The Wor1-like protein Fgp1 regulates pathogenicity, toxin synthesis and reproduction in the phytopathogenic fungus Fusarium graminearum
- PMID: 22693448
- PMCID: PMC3364952
- DOI: 10.1371/journal.ppat.1002724
The Wor1-like protein Fgp1 regulates pathogenicity, toxin synthesis and reproduction in the phytopathogenic fungus Fusarium graminearum
Abstract
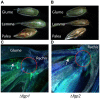
WOR1 is a gene for a conserved fungal regulatory protein controlling the dimorphic switch and pathogenicity determents in Candida albicans and its ortholog in the plant pathogen Fusarium oxysporum, called SGE1, is required for pathogenicity and expression of key plant effector proteins. F. graminearum, an important pathogen of cereals, is not known to employ switching and no effector proteins from F. graminearum have been found to date that are required for infection. In this study, the potential role of the WOR1-like gene in pathogenesis was tested in this toxigenic fungus. Deletion of the WOR1 ortholog (called FGP1) in F. graminearum results in greatly reduced pathogenicity and loss of trichothecene toxin accumulation in infected wheat plants and in vitro. The loss of toxin accumulation alone may be sufficient to explain the loss of pathogenicity to wheat. Under toxin-inducing conditions, expression of genes for trichothecene biosynthesis and many other genes are not detected or detected at lower levels in Δfgp1 strains. FGP1 is also involved in the developmental processes of conidium formation and sexual reproduction and modulates a morphological change that accompanies mycotoxin production in vitro. The Wor1-like proteins in Fusarium species have highly conserved N-terminal regions and remarkably divergent C-termini. Interchanging the N- and C- terminal portions of proteins from F. oxysporum and F. graminearum resulted in partial to complete loss of function. Wor1-like proteins are conserved but have evolved to regulate pathogenicity in a range of fungi, likely by adaptations to the C-terminal portion of the protein.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
-
- Goswami RS, Kistler HC. Heading for disaster: Fusarium graminearum on cereal crops. Mol Plant Pathol. 2004;5:515–525. - PubMed
-
- Ilgen P, Hadeler B, Maier FJ, Schafer W. Developing kernel and rachis node induce the trichothecene pathway of Fusarium graminearum during wheat head infection. Mol Plant Microbe Interact. 2009;22:899–908. - PubMed
-
- Kimura M, Tokai T, Takahashi-Ando N, Ohsato S, Fujimura M. Molecular and genetic studies of fusarium trichothecene biosynthesis: pathways, genes, and evolution. Biosci Biotechnol Biochem. 2007;71:2105–2123. - PubMed

