Potential of host markers produced by infection phase-dependent antigen-stimulated cells for the diagnosis of tuberculosis in a highly endemic area
- PMID: 22693640
- PMCID: PMC3367928
- DOI: 10.1371/journal.pone.0038501
Potential of host markers produced by infection phase-dependent antigen-stimulated cells for the diagnosis of tuberculosis in a highly endemic area
Erratum in
- PLoS One. 2012;7(8). doi:10.1371/annotation/bc36a9c6-d5c0-4d55-bc92-9ce4a07b4f70
Abstract
Background: Recent interferon gamma (IFN-γ)-based studies have identified novel Mycobacterium tuberculosis (M.tb) infection phase-dependent antigens as diagnostic candidates. In this study, the levels of 11 host markers other than IFN-γ, were evaluated in whole blood culture supernatants after stimulation with M.tb infection phase-dependent antigens, for the diagnosis of TB disease.
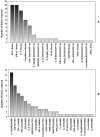
Methodology and principal findings: Five M.tb infection phase-dependent antigens, comprising of three DosR-regulon-encoded proteins (Rv2032, Rv0081, Rv1737c), and two resucitation promoting factors (Rv0867c and Rv2389c), were evaluated in a case-control study with 15 pulmonary TB patients and 15 household contacts that were recruited from a high TB incidence setting in Cape Town, South Africa. After a 7-day whole blood culture, supernatants were harvested and the levels of the host markers evaluated using the Luminex platform. Multiple antigen-specific host markers were identified with promising diagnostic potential. Rv0081-specific levels of IL-12(p40), IP-10, IL-10 and TNF-α were the most promising diagnostic candidates, each ascertaining TB disease with an accuracy of 100%, 95% confidence interval for the area under the receiver operating characteristics plots, (1.0 to 1.0).
Conclusions: Multiple cytokines other than IFN-γ in whole blood culture supernatants after stimulation with M.tb infection phase-dependent antigens show promise as diagnostic markers for active TB. These preliminary findings should be verified in well-designed diagnostic studies employing short-term culture assays.
Conflict of interest statement
Figures





References
-
- Chegou NN, Hoek KG, Kriel M, Warren RM, Victor TC, et al. Tuberculosis assays: past, present and future. Expert Rev Anti Infect Ther. 2011;9:457–469. - PubMed
-
- Trebucq A, Enarson DA, Chiang CY, Van DA, Harries AD, et al. Xpert((R)) MTB/RIF for national tuberculosis programmes in low-income countries: when, where and how? Int J Tuberc Lung Dis. 2011;15:1567–1572. - PubMed
-
- Chegou NN, Walzl G, Bolliger CT, Diacon AH, van den Heuvel MM. Evaluation of adapted whole-blood interferon-gamma release assays for the diagnosis of pleural tuberculosis. Respiration. 2008;76:131–138. - PubMed
-
- Munk ME, Arend SM, Brock I, Ottenhoff TH, Andersen P. Use of ESAT-6 and CFP-10 antigens for diagnosis of extrapulmonary tuberculosis. J Infect Dis. 2001;183:175–176. - PubMed
-
- Diel R, Goletti D, Ferrara G, Bothamley G, Cirillo D, et al. Interferon-gamma release assays for the diagnosis of latent Mycobacterium tuberculosis infection: a systematic review and meta-analysis. Eur Respir J. 2011;37:88–99. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

