Photocontrolled binding and binding-controlled photochromism within anthracene-modified DNA
- PMID: 22694485
- PMCID: PMC3614019
- DOI: 10.1021/ja304205m
Photocontrolled binding and binding-controlled photochromism within anthracene-modified DNA
Abstract
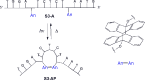
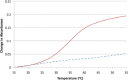
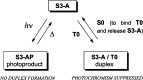
Modified DNA strands undergo a reversible light-induced reaction involving the intramolecular photodimerization of two appended anthracene tags. The photodimers exhibit markedly different binding behavior toward a complementary strand that depends on the number of bases between the modified positions. By preforming the duplex, photochromism can be suppressed, illustrating dual-mode gated behavior.
Figures
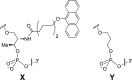


References
-
-
For some recent reviews and examples, see:
- Sortino S. J. Mater. Chem. 2012, 22, 301.
- Mok A. K.; Kedzierski N. A.; Chung P. N.; Lukeman P. S. Chem. Commun. 2011, 47, 4905.and references therein. - PubMed
- Lee H.-M.; Larson D. R.; Lawrence D. S. ACS Chem. Biol. 2009, 4, 409. - PMC - PubMed
- Mayer G.; Heckel A. Angew. Chem., Int. Ed. 2006, 45, 4900. - PubMed
- For a recent special issue on DNA nanotechnology, see:Stulz E.; Clever G.; Shionoya M.; Mao C. Chem. Soc. Rev. 2011, 40, 5633. - PubMed
-
-
-
For examples of azobenzene systems, see:
- Nishioka H.; Liang X.; Kato T.; Asanuma H. Angew. Chem., Int. Ed. 2012, 51, 1165.and references therein. - PubMed
- Kashida H.; Liang X.; Asanuma H. Curr. Org. Chem. 2009, 13, 1065.
- Liu M. Z.; Asanuma H.; Komiyama M. J. Am. Chem. Soc. 2006, 128, 1009. - PubMed
- Stafforst T.; Hilvert D. Angew. Chem., Int. Ed. 2010, 49, 9998. - PubMed
- Keiper S.; Vyle J. S. Angew. Chem., Int. Ed. 2006, 45, 3306. - PubMed
- For a recent review of azobenzene photoswitching within biomolecules, see:Beharry A. A.; Woolley G. A. Chem. Soc. Rev. 2011, 40, 4422. - PubMed
-
-
-
For example of non-azobenzene trans–cis systems, see:
- Ogasawara S.; Maeda M. Angew. Chem., Int. Ed. 2009, 48, 6671. - PubMed
- Ogasawara S.; Maeda M. Angew. Chem., Int. Ed. 2008, 47, 8839. - PubMed
- Lewis F. D.; Liu X. J. Am. Chem. Soc. 1999, 121, 11928.
- Herrlein M. K.; Letsinger R. L. Angew. Chem., Int. Ed. Engl. 1997, 36, 599.
-
-
-
For other examples of anthracene photodimerisation in DNA, see:
- Pasternak K.; Pasternak A.; Gupta P.; Veedu R. N.; Wengel J. Biorg. Med. Chem. 2011, 19, 7407. - PubMed
- Arslan P.; Jyo A.; Ihara T. Org. Biomol. Chem. 2010, 8, 4843. - PubMed
- Mukae M.; Ihara T.; Tabara M.; Jyo A. Org. Biomol. Chem. 2009, 7, 1349. - PubMed
- Ihara T.; Fujii T.; Mukae M.; Kitamura Y.; Jyo A. J. Am. Chem. Soc. 2004, 126, 8880. - PubMed
-
-
- McSkimming G.; Tucker J. H. R.; Bouas-Laurent H.; Desvergne J.-P.; Coles S. J.; Hursthouse M. B.; Light M. E. Chem.—Eur. J. 2002, 8, 3331. - PubMed
- Molard Y.; Bassani D. M.; Desvergne J.-P.; Moran N.; Tucker J. H. R. J. Org. Chem. 2006, 71, 8523. - PubMed
- Molard Y.; Bassani D. M.; Desvergne J.-P.; Horton P. N.; Hursthouse M. B.; Tucker J. H. R. Angew. Chem., Int. Ed. 2005, 44, 1072. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

