Subclinical inflammation and chronic renal allograft injury in a randomized trial on steroid avoidance in pediatric kidney transplantation
- PMID: 22694733
- PMCID: PMC3459071
- DOI: 10.1111/j.1600-6143.2012.04144.x
Subclinical inflammation and chronic renal allograft injury in a randomized trial on steroid avoidance in pediatric kidney transplantation
Abstract
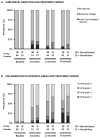
Steroid avoidance is safe and effective in children receiving kidney transplants in terms of graft function and survival, but the effects on allograft histology are unknown. In this multicenter trial, 130 pediatric renal transplant recipients were randomized to steroid-free (SF; n = 60) or steroid-based (SB; n = 70) immunosuppression, and underwent renal allograft biopsies at the time of graft dysfunction and per protocol at implantation and 6, 12 and 24 months after transplantation. Clinical follow-up was 3 years posttransplant. Subclinical acute rejection was present in 10.6% SF versus 11.3% SB biopsies at 6 months (p = 0.91), 0% SF versus 4.3% SB biopsies at 1 year (p = 0.21) and 0% versus 4.8% at 2 years (p = 0.20). Clinical acute rejection was present in 13.3% SF and 11.4% SB patients by 1 year (p = 0.74) and in 16.7% SF and 17.1% SB patients by 3 years (p = 0.94) after transplantation. The cumulative incidence of antibody-mediated rejection was 6.7% in SF and 2.9% in SB by 3 years after transplantation (p = 0.30). There was a significant increase in chronic histological damage over time (p < 0.001), without difference between SF and SB patients. Smaller recipient size and higher donor age were the main risk factors for chronic histological injury in posttransplant biopsies.
Trial registration: ClinicalTrials.gov NCT00141037.
© Copyright 2012 The American Society of Transplantation and the American Society of Transplant Surgeons.
Conflict of interest statement
None of the authors have any conflict of interest to declare in relation to this study.
Figures






Comment in
-
Transplantation: Steroid avoidance in pediatric transplant patients is safe.Nat Rev Nephrol. 2012 Jul 3;8(8):434. doi: 10.1038/nrneph.2012.130. Nat Rev Nephrol. 2012. PMID: 22751506 No abstract available.
References
-
- McDonald SP, Craig JC. Long-term survival of children with end-stage renal disease. N Engl J Med. 2004 Jun 24;350(26):2654–62. - PubMed
-
- Nankivell B, Richard J, Borrows R, Fung CL-S, O’Connell PJ, Allen R, et al. The natural history of chronic allograft nephropathy. New Engl J Med. 2003;349(24):2326–33. - PubMed
-
- El-Zoghby ZM, Stegall MD, Lager DJ, Kremers WK, Amer H, Gloor JM, et al. Identifying specific causes of kidney allograft loss. American Journal of Transplantation. 2009 Mar;9(3):527–35. - PubMed
-
- Naesens M, Kambham N, Concepcion W, Salvatierra O, Jr, Sarwal M. The evolution of non-immune histological injury and its clinical relevance in adult-sized kidney grafts in pediatric recipients. Am J Transplant. 2007;7(11):2504–14. - PubMed
-
- Chapman JR, O’Connell PJ, Nankivell BJ. Chronic renal allograft dysfunction. J Am Soc Nephrol. 2005 Oct 1;16(10):3015–26. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

