Safety and preliminary efficacy analysis of the mTOR inhibitor ridaforolimus in patients with taxane-treated, castration-resistant prostate cancer
- PMID: 22695254
- PMCID: PMC3963491
- DOI: 10.1016/j.clgc.2012.05.001
Safety and preliminary efficacy analysis of the mTOR inhibitor ridaforolimus in patients with taxane-treated, castration-resistant prostate cancer
Abstract
Background: Few options are available after taxane-based therapy in men with CRPC. Genetic alterations involving the mTOR pathway have been associated with CRPC development, raising the hypothesis that blocking mTOR signaling may be an effective targeted approach to treatment.
Patients and methods: In this open-label phase II study, the mTOR inhibitor Ridaforolimus was administered at a dose of 50 mg intravenous once weekly to 38 patients with taxane-treated CRPC. The primary end point was best overall response according to modified Response Evaluation Criteria in Solid Tumors guidelines. Serum prostate-specific antigen levels were prospectively monitored as a biomarker for cancer activity.
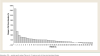
Results: No objective responses were observed, but 18 patients (47.4%) had stable disease as their best response. Based on progression-free survival analysis, median time to progression with Ridaforolimus was 28 days (95% confidence interval, 27-29). Eight patients (21.1%) had stable disease as their best overall prostate-specific antigen response. The median number of days from first to last dose was 109.5 days (range, 1-442 days). Ridaforolimus was generally well tolerated, with a safety profile similar to that observed in patients with advanced malignancies. The most common side effects were typically mild or moderate in severity.
Conclusions: Ridaforolimus was generally well tolerated. Treatment did not produce objective responses, but stable disease was observed in some patients with taxane-treated CRPC. Alternative treatment regimens, such as combination therapy with a taxane or in a maintenance treatment paradigm, should be considered for further evaluation in this patient population.
Trial registration: ClinicalTrials.gov NCT00110188.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures


References
-
- American Cancer Society . Cancer Facts and Figures 2010. American Cancer Society; Atlanta: 2010.
-
- Surveillance Epidemiology and End Results Program [May 29, 2012];SEER Stat Fact Sheets: Prostate. Available at: http://seer.cancer.gov/statfacts/html/prost.html.
-
- National Comprehensive Cancer Network [May 29, 2012];NCCN clinical practice guidelines in oncology: prostate cancer. Available at: http://www.nccn.org/professionals/physician_gls/f_guidelines.asp#prostate.
-
- Petrylak DP, Tangen CM, Hussain MH, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351:1513–20. - PubMed
-
- Tannock IF, de Wit R, Berry WR, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351:1502–12. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

