Isoniazid pharmacokinetics, pharmacodynamics, and dosing in South African infants
- PMID: 22695364
- PMCID: PMC3397663
- DOI: 10.1097/FTD.0b013e31825c4bc3
Isoniazid pharmacokinetics, pharmacodynamics, and dosing in South African infants
Abstract
Aims: There are limited data on isoniazid (INH) pharmacokinetics in infants and young children and, therefore, uncertainty on appropriate dosing.
Methods: Pharmacokinetic data were obtained from perinatally HIV-exposed South African infants aged 3-24 months receiving INH 10-20 mg·kg·d orally for Mycobacterium tuberculosis prophylaxis. INH pharmacokinetic parameters were characterized using a population pharmacokinetic approach. Dosing simulations were performed to evaluate weight-based INH doses in children based on N-acetyltransferase 2 enzyme (NAT2) genotype, age, maximum concentrations (Cmax) ≥3 mg/L, and area under the curve (AUC0-24) ≥10.52 mg·h/L.
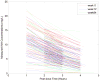
Results: In 151 infants (53% female, 48% HIV positive) receiving a mean INH dose of 14.5 mg·kg·d, mean (±SD) Cmax at 3, 6, and 23 months of age were 10.0 (3.5), 8.6 (2.6), and 9.3 (3.8) mg/L, respectively, mean (±SD) AUC0-24 were 53.6 (26.8), 42 (19.9), and 44 (30.7) mg·h/L, respectively, and mean (±SD) half-lives were 2.1 (0.7), 1.9 (0.6), and 1.8 (0.9) hours, respectively. A trimodal apparent oral clearance of INH as a function of the NAT2 genotype was apparent as early as 3 months. INH was well tolerated. At an average INH dose of 14.5 mg·kg·d, 99% of infants aged 3-24 months have an INH Cmax ≥3 mg/L, and 98% have an INH AUC0-24 ≥10.52 mg·h/L.
Conclusions: INH at an average dose of 14.5 mg/kg once daily was well tolerated in infants and achieved INH Cmax values ≥3 mg/L and AUC0-24 values ≥10.52 mg·h/L.
Figures
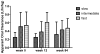
References
-
- Swaminathan S, Rekha B. Pediatric tuberculosis: global overview and challenges. Clin Infect Dis. 2010;50(Suppl 3):S184–94. - PubMed
-
- Weber WW, Hein DW. Clinical pharmacokinetics of isoniazid. Clinical pharmacokinetics. 1979;4(6):401–22. - PubMed
-
- Walker K, Ginsberg G, Hattis D, et al. Genetic polymorphism in N-Acetyltransferase (NAT): Population distribution of NAT1 and NAT2 activity. Journal of toxicology and environmental health. 2009;12(5–6):440–72. - PubMed
-
- Preziosi P. Isoniazid: metabolic aspects and toxicological correlates. Current drug metabolism. 2007;8(8):839–51. - PubMed
-
- Donald PR, Sirgel FA, Venter A, et al. The influence of human N-acetyltransferase genotype on the early bactericidal activity of isoniazid. Clin Infect Dis. 2004;39(10):1425–30. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

