Casein kinase 2α regulates multidrug resistance-associated protein 1 function via phosphorylation of Thr249
- PMID: 22695718
- PMCID: PMC3422697
- DOI: 10.1124/mol.112.078295
Casein kinase 2α regulates multidrug resistance-associated protein 1 function via phosphorylation of Thr249
Abstract
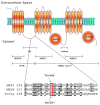
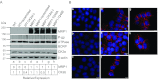
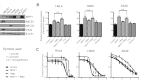
We have shown previously that the function of Ycf1p, yeast ortholog of multidrug resistance-associated protein 1 (MRP1), is regulated by yeast casein kinase 2α (Cka1p) via phosphorylation at Ser251. In this study, we explored whether casein kinase 2α (CK2α), the human homolog of Cka1p, regulates MRP1 by phosphorylation at the semiconserved site Thr249. Knockdown of CK2α in MCF7-derived cells expressing MRP1 [MRP1 CK2α(-)] resulted in increased doxorubicin sensitivity. MRP1-dependent transport of leukotriene C(4) and estradiol-17β-d-glucuronide into vesicles derived from MRP1 CK2α(-) cells was decreased compared with MRP1 vesicles. Moreover, mutation of Thr249 to alanine (MRP1-T249A) also resulted in decreased MRP1-dependent transport, whereas a phosphomimicking mutation (MRP1-T249E) led to dramatic increase in MRP1-dependent transport. Studies in tissue culture confirmed these findings, showing increased intracellular doxorubicin accumulation in MRP1 CK2α(-) and MRP1-T249A cells compared with MRP1 cells. Inhibition of CK2 kinase by 2-dimethylamino-4,5,6,7-tetrabromo-1H-benzimidazole resulted in increased doxorubicin accumulation in MRP1 cells, but not in MRP1 CK2α(-), MRP1-T249A, or MRP1-T249E cells, suggesting that CK2α regulates MRP1 function via phosphorylation of Thr249. Indeed, CK2α and MRP1 interact physically, and recombinant CK2 phosphorylates MRP1-derived peptide in vitro in a Thr249-dependent manner, whereas knockdown of CK2α results in decreased phosphorylation at MRP1-Thr249. The role of CK2 in regulating MRP1 was confirmed in other cancer cell lines where CK2 inhibition decreased MRP1-mediated efflux of doxorubicin and increased doxorubicin cytotoxicity. This study supports a model in which CK2α potentiates MRP1 function via direct phosphorylation of Thr249.
Figures



Similar articles
-
Suppression of Ycf1p function by Cka1p-dependent phosphorylation is attenuated in response to salt stress.FEMS Yeast Res. 2010 Nov;10(7):839-57. doi: 10.1111/j.1567-1364.2010.00677.x. Epub 2010 Aug 31. FEMS Yeast Res. 2010. PMID: 20812950 Free PMC article.
-
A naturally occurring mutation in MRP1 results in a selective decrease in organic anion transport and in increased doxorubicin resistance.Pharmacogenetics. 2002 Jun;12(4):321-30. doi: 10.1097/00008571-200206000-00008. Pharmacogenetics. 2002. PMID: 12042670
-
Influence of casein kinase II in tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis in human rhabdomyosarcoma cells.Clin Cancer Res. 2004 Oct 1;10(19):6650-60. doi: 10.1158/1078-0432.CCR-04-0576. Clin Cancer Res. 2004. PMID: 15475455
-
Targeting multidrug resistance protein 1 (MRP1, ABCC1): past, present, and future.Annu Rev Pharmacol Toxicol. 2014;54:95-117. doi: 10.1146/annurev-pharmtox-011613-135959. Epub 2013 Sep 18. Annu Rev Pharmacol Toxicol. 2014. PMID: 24050699 Review.
-
Multidrug resistance associated proteins in multidrug resistance.Chin J Cancer. 2012 Feb;31(2):58-72. doi: 10.5732/cjc.011.10329. Epub 2011 Nov 18. Chin J Cancer. 2012. PMID: 22098952 Free PMC article. Review.
Cited by
-
The structural basis for regulation of the glutathione transporter Ycf1 by regulatory domain phosphorylation.Nat Commun. 2022 Mar 11;13(1):1278. doi: 10.1038/s41467-022-28811-w. Nat Commun. 2022. PMID: 35277487 Free PMC article.
-
Role of protein kinase CK2 in antitumor drug resistance.J Exp Clin Cancer Res. 2019 Jul 5;38(1):287. doi: 10.1186/s13046-019-1292-y. J Exp Clin Cancer Res. 2019. PMID: 31277672 Free PMC article. Review.
-
Structural basis for the modulation of MRP2 activity by phosphorylation and drugs.Nat Commun. 2024 Mar 4;15(1):1983. doi: 10.1038/s41467-024-46392-8. Nat Commun. 2024. PMID: 38438394 Free PMC article.
-
Knowledge-guided gene prioritization reveals new insights into the mechanisms of chemoresistance.Genome Biol. 2017 Aug 11;18(1):153. doi: 10.1186/s13059-017-1282-3. Genome Biol. 2017. PMID: 28800781 Free PMC article.
-
Post-translational modifications of transporters.Pharmacol Ther. 2018 Dec;192:88-99. doi: 10.1016/j.pharmthera.2018.06.013. Epub 2018 Jun 30. Pharmacol Ther. 2018. PMID: 29966598 Free PMC article. Review.
References
-
- Ausubel FM, Brent R, Kingston RE, Moore FF, Seidman JG, Smith JA, Struhl K. (1987) Current Protocols in Molecular Biology, Greene Publishing Associates/Wiley Interscience, New York
-
- Bakos E, Evers R, Calenda G, Tusnády GE, Szakács G, Váradi A, Sarkadi B. (2000) Characterization of the amino-terminal regions in the human multidrug resistance protein (MRP1). J Cell Sci 113:4451–4461 - PubMed
-
- Burnett G, Kennedy EP. (1954) The enzymatic phosphorylation of proteins. J Biol Chem 211:969–980 - PubMed
-
- Campling BG, Pym J, Galbraith PR, Cole SP. (1988) Use of the MTT assay for rapid determination of chemosensitivity of human leukemic blast cells. Leuk Res 12:823–831 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

