Chemoselective hydroxyl group transformation: an elusive target
- PMID: 22695722
- PMCID: PMC3430791
- DOI: 10.1039/c2mb25122a
Chemoselective hydroxyl group transformation: an elusive target
Abstract
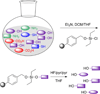
The selective reaction of one functional group in the presence of others is not a trivial task. A noteworthy amount of research has been dedicated to the chemoselective reaction of the hydroxyl moiety. This group is prevalent in many biologically important molecules including natural products and proteins. However, targeting the hydroxyl group is difficult for many reasons including its relatively low nucleophilicity in comparison to other ubiquitous functional groups such as amines and thiols. Additionally, many of the developed chemoselective reactions cannot be used in the presence of water. Despite these complications, chemoselective transformation of the hydroxyl moiety has been utilized in the synthesis of complex natural product derivatives, the reaction of tyrosine residues in proteins, the isolation of natural products and is the mechanism of action of myriad drugs. Here, methods for selective targeting of this group, as well as applications of several devised methods, are described.
Figures


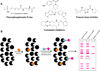
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

