Ursodeoxycholic acid versus placebo, and early term delivery versus expectant management, in women with intrahepatic cholestasis of pregnancy: semifactorial randomised clinical trial
- PMID: 22695903
- PMCID: PMC3420230
- DOI: 10.1136/bmj.e3799
Ursodeoxycholic acid versus placebo, and early term delivery versus expectant management, in women with intrahepatic cholestasis of pregnancy: semifactorial randomised clinical trial
Abstract
Objectives: To test whether ursodeoxycholic acid reduces pruritus in women with intrahepatic cholestasis of pregnancy, whether early term delivery does not increase the incidence of caesarean section, and the feasibility of recruiting women with intrahepatic cholestasis of pregnancy to trials of these interventions.
Design: First phase of a semifactorial randomised controlled trial.
Setting: Nine consultant led maternity units, United Kingdom.
Participants: 125 women with intrahepatic cholestasis of pregnancy (pruritus and raised levels of serum bile acids) or pruritus and raised alanine transaminase levels (>100 IU/L) recruited after 24 weeks' gestation and followed until delivery. 56 women were randomised to ursodeoxycholic acid, 55 to placebo, 30 to early term delivery, and 32 to expectant management.
Interventions: Ursodeoxycholic acid 500 mg twice daily or placebo increased as necessary for symptomatic or biochemical improvement until delivery; early term delivery (induction or delivery started between 37+0 and 37+6) or expectant management (spontaneous labour awaited until 40 weeks' gestation or caesarean section undertaken by normal obstetric guidelines, usually after 39 weeks' gestation).
Main outcome measures: The primary outcome for ursodeoxycholic acid was maternal itch (arithmetic mean of measures (100 mm visual analogue scale) of worst itch in past 24 hours) and for the timing of delivery was caesarean section. Secondary outcomes were other maternal and perinatal outcomes and recruitment rates.
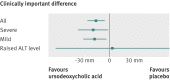
Results: Ursodeoxycholic acid reduced itching by -16 mm (95% confidence interval -27 mm to -6 mm), less than the 30 mm difference prespecified by clinicians and women as clinically meaningful. 32% (14/44) of women randomised to ursodeoxycholic acid experienced a reduction in worst itching by at least 30 mm compared with 16% (6/37) randomised to placebo. The difference of 16% (95% confidence interval -3 to 34); this would represent a number needed to treat of 6, but it failed to reach significance. Early term delivery did not increase caesarean sections (7/30 (23%) in the early term delivery group versus 11/32 (33%) in the expectant management group (relative risk 0.70, 95% confidence interval 0.31 to 1.57). No serious harms were noted in either trial. 22% (73/325) of eligible women participated in the drug trial and 19% (39/209) in the timing of delivery trial; both groups had a similar spectrum of disease severity to non-participants.
Conclusions: Ursodeoxycholic acid significantly reduces pruritus, but the size of the benefit may be too small for most doctors to recommend it, or for most women to want to take it. Women are, however, likely to differ in whether they consider the benefit to be worthwhile. Planned early term delivery seems not to increase incidence of caesarean section, although a small increase cannot be excluded. A trial to test whether ursodeoxycholic acid reduces adverse perinatal outcomes would have to be large, but is feasible. A trial to test the effect of early term delivery on adverse fetal outcomes would have to be significantly larger and may not be feasible.
Trial registration: Current Controlled Trials ISRCTN37730443.
Conflict of interest statement
Competing interests: All authors have completed the ICMJE uniform disclosure form at
Figures


References
-
- Fisk NM, Bye WB, Storey GN. Maternal features of obstetric cholestasis: 20 years experience at King George V Hospital. Aust N Z J Obstet Gynaecol 1988;28:172-6. - PubMed
-
- Saleh MM, Abdo KR. Intrahepatic cholestasis of pregnancy: review of the literature and evaluation of current evidence. J Womens Health (Larchmt) 2007;16:833-41. - PubMed
-
- Brites D, Rodrigues CM, Cardoso Mda C, Graca LM. Unusual case of severe cholestasis of pregnancy with early onset, improved by ursodeoxycholic acid administration. Eur J Obstet Gynecol Reprod Biol 1998;76:165-8. - PubMed
-
- Abedin P, Weaver JB, Egginton E. Intrahepatic cholestasis of pregnancy: prevalence and ethnic distribution. Ethn Health 1999;4:35-7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
