Cyclic AMP signaling in bone marrow stromal cells has reciprocal effects on the ability of mesenchymal stem cells to differentiate into mature osteoblasts versus mature adipocytes
- PMID: 22695986
- PMCID: PMC3509326
- DOI: 10.1007/s12020-012-9717-9
Cyclic AMP signaling in bone marrow stromal cells has reciprocal effects on the ability of mesenchymal stem cells to differentiate into mature osteoblasts versus mature adipocytes
Abstract
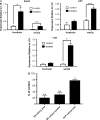
Stimulatory G protein-mediated cAMP signaling is intimately involved in skeletal homeostasis. However, limited information is available on the role of the cAMP signaling in regulating the differentiation of mesenchymal stem cells into mature osteoblasts and adipocytes. To investigate this, we treated primary mouse bone marrow stromal cells (BMSCs) with forskolin to stimulate cAMP signaling and determined the effect on osteoblast and adipocyte differentiation. Exposure of differentiating osteoblasts to forskolin markedly inhibited progression to the late stages of osteoblast differentiation, and this effect was replicated by continuous exposure to PTH. Strikingly, forskolin activation of cAMP signaling in BMSCs conditioned mesenchymal stem cells (MSCs) to undergo increased osteogenic differentiation and decreased adipogenic differentiation. PTH treatment of BMSCs also enhanced subsequent osteogenesis, but promoted an increased adipogenesis as well. Thus, activation of cAMP signaling alters the lineage commitment of MSCs, favoring osteogenesis at the expense of adipogenesis.
Figures







References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

