Quantification of iron concentration in the liver by MRI
- PMID: 22696043
- PMCID: PMC3314738
- DOI: 10.1007/s13244-011-0132-1
Quantification of iron concentration in the liver by MRI
Abstract
Objective: Measurement of liver iron concentration is a key parameter for the management of patients with primary and secondary haemochromatosis. Magnetic resonance imaging (MRI) has already demonstrated high accuracy to quantify liver iron content. To be able to improve the current management of patients that are found to have iron overload, we need a reproducible, standardised method that is, or can easily be made, widely available.
Methods: This article discusses the different MRI techniques and models to quantify liver iron concentration that are currently available and envisaged for the near future from a realistic perspective.
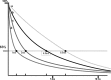
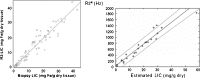
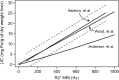
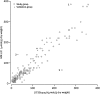
Results: T2 relaxometry methods are more accurate than signal intensity ratio (SIR) methods and they are reproducible but are not yet standardised or widely available. SIR methods, on the other hand, are very specific for all levels of iron overload and, what is more, they are also reproducible, standardised and already widely available.
Conclusions: For these reasons, today, both methods remain necessary while progress is made towards universal standardisation of the relaxometry technique.
Figures

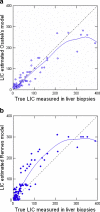
References
LinkOut - more resources
Full Text Sources

