Regimens of fetal surveillance for impaired fetal growth
- PMID: 22696366
- PMCID: PMC6465035
- DOI: 10.1002/14651858.CD007113.pub3
Regimens of fetal surveillance for impaired fetal growth
Abstract
Background: Policies and protocols for fetal surveillance in the pregnancy where impaired fetal growth is suspected vary widely, with numerous combinations of different surveillance methods.
Objectives: To assess the effects of antenatal fetal surveillance regimens on important perinatal and maternal outcomes.
Search methods: We searched the Cochrane Pregnancy and Childbirth Group's Trials Register (29 February 2012).
Selection criteria: Randomised and quasi-randomised trials comparing the effects of described antenatal fetal surveillance regimens.
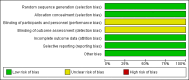
Data collection and analysis: Review authors R Grivell and L Wong independently assessed trial eligibility and quality and extracted data.
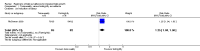
Main results: We included one trial of 167 women and their babies. This trial was a pilot study recruiting alongside another study, therefore, a separate sample size was not calculated. The trial compared a twice-weekly surveillance regimen (biophysical profile, nonstress tests, umbilical artery and middle cerebral artery Doppler and uterine artery Doppler) with the same regimen applied fortnightly (both groups had growth assessed fortnightly). There were insufficient data to assess this review's primary infant outcome of composite perinatal mortality and serious morbidity (although there were no perinatal deaths) and no difference was seen in the primary maternal outcome of emergency caesarean section for fetal distress (risk ratio (RR) 0.96; 95% confidence interval (CI) 0.35 to 2.63). In keeping with the more frequent monitoring, mean gestational age at birth was four days less for the twice-weekly surveillance group compared with the fortnightly surveillance group (mean difference (MD) -4.00; 95% CI -7.79 to -0.21). Women in the twice-weekly surveillance group were 25% more likely to have induction of labour than those in the fortnightly surveillance group (RR 1.25; 95% CI 1.04 to 1.50).
Authors' conclusions: There is limited evidence from randomised controlled trials to inform best practice for fetal surveillance regimens when caring for women with pregnancies affected by impaired fetal growth. More studies are needed to evaluate the effects of currently used fetal surveillance regimens in impaired fetal growth.
Conflict of interest statement
None known.
Figures










Update of
-
Regimens of fetal surveillance for impaired fetal growth.Cochrane Database Syst Rev. 2009 Jan 21;(1):CD007113. doi: 10.1002/14651858.CD007113.pub2. Cochrane Database Syst Rev. 2009. Update in: Cochrane Database Syst Rev. 2012 Jun 13;(6):CD007113. doi: 10.1002/14651858.CD007113.pub3. PMID: 19160321 Free PMC article. Updated.
References
References to studies included in this review
McCowan 2000 {published data only}
-
- McCowan LME, Harding JE, Roberts AB, Barker SE, Ford C, Stewart AW. A pilot randomized controlled trial of two regimens of fetal surveillance for small‐for‐gestational‐age fetuses with normal results of umbilical artery Doppler velocimetry. American Journal of Obstetrics and Gynecology 2000;182(1):81‐6. - PubMed
References to studies excluded from this review
Sood 2007 {published data only}
-
- Sood AK. Vibroacoustic stimulation and modified fetal biophysical profile in high risk pregnancy. Journal of Obstetrics and Gynaecology of India 2007;57(1):27‐36.
Williams 2003 {published data only}
-
- Williams KP, Farquharson DF, Bebbington M, Dansereau J, Galerneau F, Wilson RD, et al. Screening for fetal well‐being in a high‐risk population comparing the nonstress test with umbilical artery Doppler velocimetry: a randomized controlled trial. American Journal of Obstetrics and Gynecology 2003;188:1366‐71. - PubMed
Additional references
Alfirevic 2010
Baschat 2001
-
- Baschat AA, Harman CR. Antenatal assessment of the growth restricted fetus. Current Opinion in Obstetrics and Gynaecology 2001;13:161‐8. - PubMed
Baschat 2005
-
- Baschat AA. Arterial and venous Doppler in the diagnosis and management of early onset fetal growth restriction. Early Human Development 2005;81(11):877‐87. - PubMed
Baschat 2006
-
- Baschat AA. Fetal growth disorders. High Risk Pregnancy Management Options. Philadelphia: Saunders, 2006.
Breeze 2007
-
- Breeze AC, Lees CC. Prediction and perinatal outcomes of fetal growth restriction. Seminars in Fetal and Neonatal Medicine 2007;12(5):363‐97. - PubMed
Deeks 2001
-
- Deeks JJ, Altman DG, Bradburn MJ. Statistical methods for examining heterogeneity and combining results from several studies in meta‐analysis. In: Egger M, Davey Smith G, Altman DG editor(s). Systematic reviews in health care: meta‐analysis in context. London: BMJ Books, 2001.
Egger 1997
Gardosi 1998
-
- Gardosi J, Mul T, Mongelli M, Fagan D. Analysis of birthweight and gestational age in antepartum stillbirths. British Journal of Obstetrics and Gynaecology 1998;105(5):524‐30. - PubMed
Grivell 2010
Harbord 2006
-
- Harbord RM, Egger M, Sterne JA. A modified test for small‐study effects in meta‐analyses of controlled trials with binary endpoints. Statistics in Medicine 2006;25(20):3443‐57. - PubMed
Higgins 2008
-
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.0 [updated February 2008]. The Cochrane Collaboration, 2008. Available from www.cochrane‐handbook.org.
Higgins 2011
-
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Lalor 2008
Lees 2005
-
- Lees C, Baumgartner H. The TRUFFLE study‐‐a collaborative publicly funded project from concept to reality: how to negotiate an ethical, administrative and funding obstacle course in the European Union. Ultrasound in Obstetrics and Gynecology 2005;25(2):105‐7. - PubMed
RevMan 2008 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.0. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2008.
RevMan 2011 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Yanney 2004
-
- Yanney M, Marlow N. Paediatric consequences of fetal growth restriction. Seminars in Fetal and Neonatal Medicine 2004;9(5):411‐8. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

