Paliperidone palmitate for schizophrenia
- PMID: 22696377
- PMCID: PMC12145955
- DOI: 10.1002/14651858.CD008296.pub2
Paliperidone palmitate for schizophrenia
Abstract
Background: Paliperidone palmitate, a long-acting, intramuscular formulation of paliperidone, is now available for clinical use. Paliperidone is an active metabolite of risperidone and it is also available in an oral formulation for daily use.
Objectives: To compare the effects of paliperidone palmitate with any other treatment for people with schizophrenia and schizophrenia-like illnesses.
Search methods: We searched the Cochrane Schizophrenia Group's Register (November 2009) and inspected references of identified studies for further trials. We contacted the manufacturers of paliperidone palmitate, the Food and Drug Administration, and authors of relevant trials for additional material.
Selection criteria: We included randomised controlled trials (RCTs).
Data collection and analysis: We independently selected and critically appraised studies, extracted data and analysed on an intention-to-treat basis. Where possible and appropriate, we calculated risk ratios (RR) and their 95% confidence intervals (CI) with the number needed to benefit/harm statistic (NNB/H). We calculated mean differences (MD) for continuous data.
Main results: Five studies with 2215 participants compared paliperidone palmitate with placebo. Fewer people left studies early if they were randomised to paliperidone palmitate (n = 2183, 5 RCTs, RR 0.76 CI 0.70 to 0.84, NNTB 9 CI 7 to 14) and those receiving any dose of paliperidone palmitate were significantly less likely to show no improvement in global state (n = 1696, 4 RCTs, RR 0.79 CI 0.74 to 0.85, NNTB 7 CI 5 to 9). People randomised to paliperidone palmitate were less likely to experience a recurrence of psychosis (n = 312, 1 RCT, RR 0.28 CI 0.17 to 0.48, NNTB 5 CI 4 to 6) than those allocated to placebo in a single trial specifically designed to study recurrence. In the other studies where recurrence was recorded only as an adverse event, we found that people who received paliperidone palmitate were also less likely to experience a recurrence of psychotic symptoms (n = 1837, 4 RCTs, RR 0.55 CI 0.44 to 0.68, NNTB 10 CI 8 to 14). Paliperidone palmitate was associated with fewer reports of agitation or aggression (n = 2180, 5 RCTs, RR 0.65 CI 0.46 to 0.91, NNTB 39 CI 25 to 150) and of using anxiolytic medications (n = 2170, 5 RCTs, RR 0.89 CI 0.83 to 0.96, NNTB 16 CI 11 to 44). A consistent, significant elevation in serum prolactin (ng/mL) was found for both men and women receiving paliperidone palmitate, but the data were too heterogenous to sum. We found no evidence of sexual dysfunction in these short-term trials. People receiving paliperidone palmitate had a significantly greater increase in weight (n = 2052, 5 RCTs, MD 1.34 CI 0.97 to 1.70) in comparison with people who received placebo.Two studies with 1969 participants compared flexibly-dosed paliperidone palmitate with flexibly-dosed risperidone long-acting injection. The mean doses of paliperidone palmitate in these trials were 73.3 and 104.6 mg every four weeks compared with risperidone long-acting injection at mean doses, respectively, of 35.3 and 31.7 mg every two weeks. We found no differences between paliperidone palmitate and risperidone long-acting injection for leaving these studies early for any reason (n = 1969, 2 RCTs, RR 1.12 CI 1.00 to 1.25). Those receiving paliperidone palmitate were statistically no more likely to have a recurrence of psychotic symptoms than those receiving risperidone long-acting injection (n = 1961, 2 RCTs, RR 1.23 CI 0.98 to 1.53). While we found no significant difference in the occurrences of deaths in the pooled trials (n = 1967, 2 RCTs, RR 3.62 CI 0.60 to 21.89), we note that a total of six deaths occurred in these two trials, with five deaths among people who received paliperidone palmitate and one death among people who received risperidone long-acting injection. Although death is the most serious of adverse events, the small number of these events in these trials makes it unclear if this finding is meaningful. We found that participants randomised to paliperidone palmitate were significantly less likely to use anticholinergic medications in these trials (n = 1587, 2 RCTs, RR 0.67 CI 0.55 to 0.82, NNTB 13 CI 10 to 24). We found no data regarding paliperidone palmitate relating to services use, quality of life, behaviour, patient satisfaction, cognitive functioning or cost.
Authors' conclusions: In short-term studies, paliperidone palmitate is an antipsychotic drug that is more efficacious than placebo. We found its adverse effects to be similar to those of its related compounds, paliperidone and risperidone, with extrapyramidal movement disorders, weight gain, and tachycardia all more common with paliperidone palmitate than placebo. While no difference was found in the incidence of reported adverse sexual outcomes, paliperidone palmitate is associated with substantial increases in serum prolactin. When flexibly dosed with a mean doses of approximately 70 to 110 mg every four weeks, paliperidone palmitate appears comparable in efficacy and tolerability to risperidone long-acting injection flexibly dosed with mean doses of approximately 35 mg every two weeks.
Conflict of interest statement
The authors received no financial consideration from any parties for the preparation of this review. Dr. Nussbaum has no interests to disclose. Dr. Stroup has been a consultant for AstraZeneca, Janssen, Lilly, and Pfizer, and has spoken at events sponsored by Lilly, Lundbeck, and Pfizer.
Figures
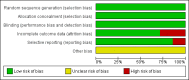










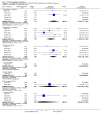









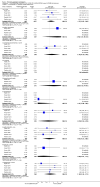

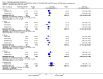





































Update of
- doi: 10.1002/14651858.CD008296
References
References to studies included in this review
Fleischhacker 2010 {published and unpublished data}
-
- Johnson and Johnson Pharmaceutical Research and Development LLC. A randomized, double blind, parallel group, comparative study of flexibly dosed paliperidone palmitate (25, 50, 75, or 100 mg eq.) administered every 4 weeks and flexibly dosed risperdal consta (25, 37.5, or 50 mg) administered every 2 weeks in subjects with schizophrenia. http://www.clinicaltrials.gov 2005. [NCT00210717]
Gopal 2010 {published and unpublished data}
-
- Gopal S, Hough DW, Xu H, Lull JM, Gassmann‐Mayer C, Remmerie BM, et al. Efficacy and safety of paliperidone palmitate in adult patients with acutely symptomatic schizophrenia: a randomized, double‐blind, placebo‐controlled, dose‐response study. International Clinical Psychopharmacology 2010;25(5):247‐56. [DOI: 10.1097/YIC.0b013e32833948fa; PUBMED: 20389255] - DOI - PubMed
-
- Johnson & Johnson Pharmaceutical Research & Development LLC. A randomized, double blind, placebo‐controlled, parallel‐group, dose‐response study to evaluate the efficacy andsafety of 3 fixed doses (50 mg eq, 100 mg eq, and 150 mg eq) of paliperidone palmitate in subjects with schizophrenia. http://www.clinicaltrials.gov 2005. [NCT00210548]
Hough 2010 {published and unpublished data}
-
- Hough D, Gopal S, Vijapurkar U, Lim P, Morozova M, Eerdekens M. Paliperidone palmitate, an atypical injectable antipsychotic, in prevention of symptom recurrence in patients with schizophrenia: a randomized, doubleblind, placebo‐controlled study. Proceedings of the 161st Annual Meeting of the American Psychiatric Association; 2008 May 3‐8; Washington DC, USA. 2008.
-
- Hough D, Gopal S, Vijapurkar U, Lim P, Morozova M, Eerdekens M. Paliperidone palmitate, an injectable antipsychotic, in prevention of symptom recurrence in patients with schizophrenia: a randomized, double‐blind, placebo‐controlled study. Biological Psychiatry. 2008; Vol. 63:285S‐6S.
-
- Hough D, Gopal S, Vijapurkar, Lim P, Morozova M, Eerdekens M. Paliperidone palmitate maintenance treatment in delaying the time‐to‐relapse in patients with schizophrenia: A randomized, double‐blind, placebo‐controlled study. Schizophrenia Research 2010;116(2‐3):107‐17. [PUBMED: 19959339] - PubMed
Kramer 2009 {published and unpublished data}
-
- Kramer M, Litman R, Hough D, Lane R, Lim P, Eerdekens M. A 9‐week, placebo‐controlled study in schizophrenia patients: efficacy and safety of the long‐acting injectable agent, paliperidone palmitate. Proceedings of the 161st Annual Meeting of the American Psychiatric Association; 2008 May 3‐8; Washington DC, USA. 2008. [Poster no. NR4‐072]
-
- Kramer M, Litman R, Hough D, Lane R, Lim P, Liu Y, et al. Paliperidone palmitate, a potential long‐acting treatment for patients with schizophrenia. Results of a randomized, double‐blind, placebo‐controlled efficacy and safety study. International Journal of Neuropsychopharmacology 2009;13(5):635‐47. - PubMed
-
- Kramer M, Litman R, Lane R, Kujawa M, Lim P, Hough D, et al. Efficacy/tolerability of paliperidone palmitate: 9‐week, placebo‐controlled study in schizophrenia patients. Schizophrenia Research 2008;98:165‐6.
-
- Kramer M, Litman R, Lane R, Lim P, Hough D, et al. Efficacy and tolerability of paliperidone palmitate: 9‐week, placebo‐controlled study in schizophrenia patients. Biological Psychiatry 2008;63:288S.
Nasrallah 2010 {published and unpublished data}
-
- Nasrallah HA, Gopal S, Gassmann‐Mayer C, Quiroz JA, Lim P, Eerdekens M, et al. A controlled, evidence‐based trial of paliperidone palmitate, a long‐acting injectable antipsychotic, in schizophrenia. Neuropsychopharmacology 2010;35(10):2072‐82. [DOI: 10.1038/npp.2010.79.; PUBMED: 20555312] - DOI - PMC - PubMed
-
- Nasrallah HA, Gopal S, Gassmann‐Mayer C, Quiroz JA, Lim P, Eerdekens M, et al. Efficacy and safety of three doses of paliperidone palmitate, an investigational long‐acting injectable antipsychotic in schizophrenia. Proceedings of the 161st Annual Meeting of the American Psychiatric Association; 2008 May 3‐8; Washington DC, USA. 2008.
Pandina 2010 {published and unpublished data}
-
- Fleischhacker WW, Gopal S, Samtani MN, Quiroz JA, Pandina G, Vermeulen A, et al. Optimization of the dosing strategy for the long‐acting injectable antipsychotic paliperidone palmitate: results of two randomized double‐blind studies and population pharmacokinetic simulations. Proceedings of the 162nd Annual Meeting of the American Psychiatric Association; 2009 May 16‐21; San Francisco, CA. 2009.
-
- Fleischhacker WW, Gopal S, Samtani MN, Quiroz JA, Pandina G, Vermeulen A, et al. Optimization of the dosing strategy for the long‐acting injectable antipsychotic paliperidone palmitate: results of two randomized double‐blind studies and population pharmacokinetic simulations. Proceedings of the 47th Annual meeting of the American College of Neuropsychopharmacology; 2008 Dec 7‐11; San Francisco, CA. 2008.
-
- Haskins JT, Sliwa JK, Ma Y‐W, Pandina GJ, Palumbo J. Efficacy and safety of 234 mg initiation dose and 3‐fixed maintenance doses of paliperidone palmitate—a once‐monthly injectable atypical antipsychotic. Proceedings of the U.S. Psychiatric and Mental Health Congress; 2009 November 2‐5; Las Vegas, Nevada. 2009.
-
- Pandina GJ, Lindenmayer J‐P, Lull J, Lim P, Gopal S, Herben V, et al. A randomized, double‐blind, placebo‐controlled, dose‐response efficacy and safety study of paliperidone palmitate in adults with schizophrenia. Proceedings of the 162nd Annual Meeting of the American Psychiatric Association; 2009 May 16‐21; San Francisco, CA. 2009.
-
- Pandina GJ, Lindenmayer JP, Lull J, Lim P, Gopal S, Herben V, et al. A randomized, placebo‐controlled study to assess the efficacy and safety of 3 doses of paliperidone palmitate in adults with acutely exacerbated schizophrenia. Journal of Clinical Psychopharmacology 2010;30(3):235‐44. [PUBMED: 20473057] - PubMed
Pandina 2011 {published and unpublished data}
-
- Fleischhacker WW, Gopal S, Samtani MN, Quiroz JA, Pandina G, Vermeulen A, et al. [Optimization of the dosing strategy for the long‐acting injectable antipsychotic paliperidone palmitate: results of two randomized double‐blind studies and population pharmacokinetic simulations]. Proceedings of the 162nd Annual Meeting of the American Psychiatric Association; 2009 May 16‐21; San Francisco, CA. 2009.
-
- Fleischhacker WW, Gopal S, Samtani MN, Quiroz JA, Pandina G, Vermeulen A, et al. Optimization of the dosing strategy for the long‐acting injectable antipsychotic paliperidone palmitate: results of two randomized double‐blind studies and population pharmacokinetic simulations. Proceedings of the 47th Annual meeting of the American College of Neuropsychopharmacology; 2008 Dec 7‐11; San Francisco, CA. 2008.
-
- Johnson & Johnson Pharmaceutical Research and Development LLC. Comparison ofpaliperidone palmitate and risperdal consta in patients with schizophrenia. http://www.clinicaltrials.gov Vol. 2007. [NCT00589914]
-
- Pandina G, Lane R, Gopal S, Gassmann‐Mayer C, Hough D, Remmerie B, et al. A double‐blind study of paliperidone palmitate and risperidone long‐acting injectable in adults with schizophrenia. Progress in Neuro‐Psychopharmacology & Biological Psychiatry 2011;35(1):218‐26. [DOI: 10.1016/j.pnpbp.2010.11.008; PUBMED: 21092748] - DOI - PubMed
References to studies excluded from this review
Cleton 2008a {published data only}
-
- Cleton A, Rossenu S, Crauwels, Berwaerts J, Hough D, Gopal S, et al. Assessment of the dose proportionality of paliperidone palmitate 25, 50, 100 and 150 mg eq., a new long‐acting injectable antipsychotic, following administration in the deltoid or gluteal muscles. Proceedings of the Institute of Psychiatric Services; 2008 October 2‐5; Chicago, Illinois. 2008.
Cleton 2008b {published data only}
-
- Cleton A, Rossenu S, Hough D, Crauwels H, Vandebosch A, Berwaerts J, et al. Evaluation of the pharmacokinetic profile of deltoid versus gluteal intramuscular injections of paliperidone palmitate in patients with schizophrenia. Institute of Psychiatric Services. Chicago, Illinois, USA, October 2‐5, 2008.
Gopal 2010b {published data only}
-
- Gopal S, Vijapurkar U, Lim P, Morozova M, Eerdekens M, Hough D. A 52‐week open‐label study of the safety and tolerability of paliperidone palmitate in patients with schizophrenia. Journal of Psychopharmacology 2010;25(5):685‐97. [PUBMED: 20615933] - PubMed
Hough 2009 {published data only (unpublished sought but not used)}
-
- Gopal S, Lindenmayer JP, Hough D, Melkote R, Lim P, Herben V, et al. Safety and tolerability of the investigational antipsychotic paliperidone palmitate injected in the deltoid or gluteus muscle in patients with schizophrenia. Proceedings of the 161st Annual Meeting of the American Psychiatric Association; 2008 May 3‐8; Washington DC, USA. 2008.
-
- Gopal S, Lindenmayer JP, Hough D, Melkote R, Lim P, Yuen E, et al. Safety and Tolerability Profiles of Paliperidone Palmitate Injected in Either the Deltoid or Gluteus Muscle in Patients with Schizophrenia. Proceedings of the Institute of Psychiatric Services; 2008 October 2‐5; Chicago, IL. 2008.
-
- Hough D, Lindenmayer JP, Gopal S, Melkote R, Lim P, Herben V, et al. Safety and tolerability of deltoid and gluteal injections of paliperidone palmitate in schizophrenia. Progress in Neuro‐Psychopharmacology and Biological Psychiatry 2009;33:1022‐31. - PubMed
Johnson and Johnson 2003b {published data only}
-
- Johnson & Johnson Pharmaceutical Research & Development LLC. Pharmacokinetics,tolerability, and safety of intramuscular injections of paliperidone palmitate in the arm or buttock of subjects with schizophrenia. http://www.clinicaltrials.gov 2003. [NCT00073320]
References to studies awaiting assessment
Li 2011 {published data only}
-
- Li H, Rui Q, Ning X, Xu H, Gu N. A comparative study of paliperidone palmitate and risperidone long‐acting injectable therapy in schizophrenia. Progress in Neuro‐Psychopharmacology & Biological Psychiatry 2011;35(4):1002‐8. [PUBMED: 21315787] - PubMed
-
- Tan Q, Shi J, Li Kq. Comparative study of paliperidone palmitate (50, 100, 150 mg eq) and risperidone LAI (long acting injection) (25, 37.5, or 50 mg) in patients with schizophrenia. http://www.clinicaltrials.gov 2008. [NCT00604279]
References to ongoing studies
Ortho 2009 {published data only (unpublished sought but not used)}
-
- Ortho‐McNeil Janssen Scientific Affairs LLC. 28‐30 month study comparing paliperidone palmitate with oral risperidone for treating adults diagnosed with schizophrenia within the past 5 years. http://www.clinicaltrials.gov 2009. [NCT00946985]
Additional references
Alphs 2010
-
- Alphs L, Gopal S, Karcher K, Kent J, Sliwa JK, Kushner S, et al. Are the long‐acting intramuscular formulations of risperidone or paliperidone palmitate associated with post‐injection delirium/sedation syndrome? an assessment of safety databases. Current Drug Safety 2010;6(1):43‐5. [PUBMED: 21047303] - PMC - PubMed
Alphs 2011
-
- Alphs L, Bossie CA, Sliwa JK, Ma YW, Turner N. Onset of efficacy with acute long‐acting injectable paliperidone palmitate treatment in markedly to severely ill patients with schizophrenia: post hoc analysis of a randomized, double‐blind clinical trial. Annals of General Psychiatry 2011;10:12. [PUBMED: 21481243] - PMC - PubMed
Altman 1996
Anonymous 2009
-
- Anon. In brief: injectable paliperidone palmitate for schizophrenia. Medical Letter 2009;51(1324):88. [PUBMED: 19890247] - PubMed
Ascher‐Svanum 2006
-
- Ascher‐Svanum H, Faries DE, Zhu B, Ernst FR, Swartz MS, Swanson JW. Medication adherence and long‐term functional outcomes in the treatment of schizophrenia in usual care. Journal of Clinical Psychiatry 2006;67(3):453‐60. [PUBMED: 16649833] - PubMed
Barnes 1989
-
- Barnes TR. A rating scale for drug‐induced akathisia. British Journal of Psychiatry 1989;154:672‐6. [MEDLINE: ] - PubMed
Bland 1997
Boissel 1999
-
- Boissel JP, Cucherat M, Li W, Chatellier G, Gueyffier F, Buyse M, et al. The problem of therapeutic efficacy indices. 3. Comparison of the indices and their use. Therapie 1999;54(4):405‐11. - PubMed
Bossie 2011
Deeks 2011
-
- Deeks JJ, Higgins JPT, Altman DG (editors). Chapter 9: Analysing data and undertaking meta‐analyses. In Cochrane Handbook for Systematic Reviews of Interventions 5.1.0 [updated March 2011] The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Divine 1992
-
- Divine GW, Brown JT, Frazier LM. The unit of analysis error in studies about physicians' patient care behavior. Journal of General Internal Medicine 1992;7(6):623‐9. [PUBMED: 1453246] - PubMed
Donner 2002
-
- Donner A, Klar N. Issues in the meta‐analysis of cluster randomized trials. Statistics in Medicine 2002;21:2971‐80. - PubMed
Egger 1997
Elbourne 2002
-
- Elbourne DR, Altman DG, Higgins JP, Curtin F, Worthington HV, Vail A. Meta‐analyses involving cross‐over trials: methodological issues. International Journal of Epidemiology 2002;31(1):140‐9. [PUBMED: 11914310] - PubMed
Elliott 2010
-
- Elliott ES, Purvis TL, Nelson LA, Sommi RW. Inconsistency in risperidone long‐acting injection steady‐state plasma levels when switching from deltoid to gluteal administration. Journal of Clinical Pharmacology 2010;50(6):721‐4. [PUBMED: 20067943] - PubMed
Fleischhacker 2009
-
- Fleischhacker WW. Second‐generation antipsychotic long‐acting injections: systematic review. British Journal of Psychiatry 2009;52(Supplement):S29‐36. [PUBMED: 19880914] - PubMed
Freedman 2003
-
- Freedman R. Schizophrenia. New England Journal of Medicine 2003;349(18):1738‐49. - PubMed
Gagnon 2006
-
- Gagnon D, Adriaenssen I, Nasrallah H, Morosini P. Reliability, validity and sensitivity to change of the personal and social performance scale in patients with stable schizophrenia. International Journal of Neuropsychopharmacology 2006;9(Suppl 1):288.
Gopal 2011
-
- Gopal S, Berwaerts J, Nuamah I, Akhras K, Coppola D, Daly E, et a. Number needed to treat and number needed to harm with paliperidone palmitate relative to long‐acting haloperidol, bromperidol, and fluphenazine decanoate for treatment of patients with schizophrenia. Neuropsychiatric Disease and Treatment 2011;8:93‐101. [PUBMED: 21552311] - PMC - PubMed
Gulliford 1999
-
- Gulliford MC, Ukoumunne OC, Chinn S. Components of variance and intraclass correlations for the design of community‐based surveys and intervention studies: Data from the health survey for England 1994. American Journal of Epidemiology 1999;149:876‐83. - PubMed
Guy 1976
-
- Guy W. Early clinical drug evaluation (ECDEU) assessment manual for psychopharmacology. Washington DC: National Institute of Mental Health, 1976.
Higgins 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hosalli 2003
Hoy 2010
-
- Hoy SM, Scott LJ, Keating GM. Intramuscular paliperidone palmitate. CNS Drugs 2010;24(3):227‐44. [PUBMED: 20155997] - PubMed
Hunter 2003
Janssen 2003
-
- Janssen Pharmaceutica Products LP. Risperdal consta (risperidone) long‐acting injection prescribing information. Janssen Pharmaceutica Products, L.P. 2003.
Janssen 2006
-
- Janssen Pharmaceutica Products LP. Invega (paliperidone) extended‐release tablets prescribing information. Janssen Pharmaceutica Products, L.P. 2006.
Janssen 2009
-
- Janssen. Invega Sustenna (paliperidone palmitate) extended‐release injectable suspension prescribing information. Orth‐McNeil‐Janssen Pharmaceuticals, Inc. 2009.
Janssen‐Cilag 1996
-
- Janssen‐Cilag Ltd. Risperidal (risperidone) summary of product characteristics. UK: Janssen‐Cilag Ltd 1996.
Kay 1986
-
- Kay S, Opler L, Fizbein A. The positive and negative syndrome scale (PANSS) manual. North Tohawanda, NY: Multi‐Health System, 1986.
Kozma 2011
-
- Kozma CM, Slaton T, Dirani R, Fastenau J, Gopal S, Hough D. Changes in schizophrenia‐related hospitalization and ER use among patients receiving paliperidone palmitate: results from a clinical trial with a 52‐week open‐label extension (OLE). Current Medical Research and Opinion 2011;27(8):1603‐11. [PUBMED: 21696265 ] - PubMed
Leucht 2005
-
- Leucht S, Kane JM, Kissling W, Hamann J, Etschel E, Engel RR. What does the PANSS mean?. Schizophrenia Research 2005;79(2‐3):231‐8. [PUBMED: 15982856] - PubMed
Leucht 2005a
-
- Leucht S, Kane JM, Kissling W, Hamann J, Etschel E, Engel R. Clinical implications of brief psychiatric rating scale scores. British Journal of Psychiatry 2005;187:366‐71. [PUBMED: 16199797] - PubMed
Lieberman 2005
-
- Lieberman JA, Stroup TS, McEvoy JP, Swartz MS, Rosenheck RA, Perkins DO, et al. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. New England Journal of Medicine 2005;353(12):1209‐23. [PUBMED: 16172203] - PubMed
Lieberman 2006
-
- Lieberman JA, Stroup TS, Perkins DO. The American Psychiatric Publishing Textbook of Schizophrenia. Washington, DC: American Psychiatric Publishing, 2006. [ISBN 1585621919]
Marder 1997
-
- Marder SR, Davis JM, Chouinard G. The effects of risperidone on the five dimensions of schizophrenia derived by factor analysis: Combined results of the North American trials. Journal of Clinical Psychiatry 1997;58(12):538‐46. [MEDLINE: ] - PubMed
Marshall 2000
-
- Marshall M, Lockwood A, Bradley C, Adams C, Joy C, Fenton M. Unpublished rating scales: a major source of bias in randomised controlled trials of treatments for schizophrenia. British Journal of Psychiatry 2000;176:249‐52. - PubMed
McEvoy 2006
-
- McEvoy JP. Risks versus benefits of different types of long‐acting injectable antipsychotics. Journal of Clinical Psychiatry 2006;67(Suppl 5):15‐8. [PUBMED: 16822092] - PubMed
Moher 2001
-
- Moher D, Schulz KF, Altman D. The CONSORT statement: revised recommendations for improving the quality of reports of parallel‐group randomized trials. JAMA 2001;285:1987‐91. - PubMed
Nussbaum 2009
Overall 1962
-
- Overall JE, Gorham DR. The brief psychiatric rating scale. Psychological Reports 1962;10:799‐812.
Patrick 2006
-
- Patrick D, Adriaenssen I, Morosini P, Rothman M. Reliability, validity and sensitivity to change of the personal and social performance scale in patients with acute schizophrenia. International Journal of Neuropsychopharmacology 2006;9(Suppl 1):S287‐8.
Patrick 2009
-
- Patrick DL, Burns T, Morosini P, Rothman M, Gagnon DD, Wild D, et al. Reliability, validity and ability to detect change of the clinician‐rated personal and social performance scale in patients with acute symptoms of schizophrenia. Current Medical Research and Opinion 2009;25(2):325‐38. [PUBMED: 19192977] - PubMed
Samtani 2009a
-
- Samtani MN, Gopal S, Sliwa JK, Haskins JT, Alphs L, Stuyckens, et al. Switching to paliperidone palmitate from other antipsychotics: guidance based on pharmacokinetic modeling and simulation. Proceedings of the 49th NCDEU: New Research Approaches for Mental Health Interventions; 2009 June 29‐ July 2; Hollywood, Florida. 2009. [http://208.81.106.111/poster%20Abstract%20Book%20final.pdf]
Samtani 2009b
Schünemann 2008
-
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In Cochrane Handbook for Systematic Reviews of Interventions 5.1.0 [updated March 2011] The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Ukoumunne 1999
-
- Ukoumunne OC, Gulliford MC, Chinn S, Sterne JAC, Burney PGJ. Methods for evaluating area‐wide and organisation‐based interventions in health and health care: a systematic review. Health Technology Assessment 1999;3(5):3‐92. - PubMed
Xia 2007
-
- Xia J, Adams CE, Bhagat N, Bhagat V, Bhoopathi P, El‐Sayeh H, et al. The Leeds Outcomes Stakeholders Survey (LOSS) Study. Proceedings of the 15th Cochrane Colloquium; 2007 Oct 23‐27; Sao Paulo. 2007.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

