Exercise and mobilisation interventions for carpal tunnel syndrome
- PMID: 22696387
- PMCID: PMC11536321
- DOI: 10.1002/14651858.CD009899
Exercise and mobilisation interventions for carpal tunnel syndrome
Abstract
Background: Non-surgical treatment, including exercises and mobilisation, has been offered to people experiencing mild to moderate symptoms arising from carpal tunnel syndrome (CTS). However, the effectiveness and duration of benefit from exercises and mobilisation for this condition remain unknown.
Objectives: To review the efficacy and safety of exercise and mobilisation interventions compared with no treatment, a placebo or another non-surgical intervention in people with CTS.
Search methods: We searched the Cochrane Neuromuscular Disease Group Specialised Register (10 January 2012), CENTRAL (2011, Issue 4), MEDLINE (January 1966 to December 2011), EMBASE (January 1980 to January 2012), CINAHL Plus (January 1937 to January 2012), and AMED (January 1985 to January 2012).
Selection criteria: Randomised or quasi-randomised controlled trials comparing exercise or mobilisation interventions with no treatment, placebo or another non-surgical intervention in people with CTS.
Data collection and analysis: Two review authors independently assessed searches and selected trials for inclusion, extracted data and assessed risk of bias of the included studies. We calculated risk ratios (RR) and mean differences (MD) with 95% confidence intervals (CIs) for primary and secondary outcomes of the review. We collected data on adverse events from included studies.
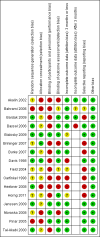
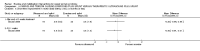
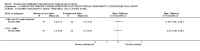
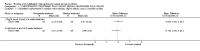
Main results: Sixteen studies randomising 741 participants with CTS were included in the review. Two compared a mobilisation regimen to a no treatment control, three compared one mobilisation intervention (for example carpal bone mobilisation) to another (for example soft tissue mobilisation), nine compared nerve mobilisation delivered as part of a multi-component intervention to another non-surgical intervention (for example splint or therapeutic ultrasound), and three compared a mobilisation intervention other than nerve mobilisation (for example yoga or chiropractic treatment) to another non-surgical intervention. The risk of bias of the included studies was low in some studies and unclear or high in other studies, with only three explicitly reporting that the allocation sequence was concealed, and four reporting blinding of participants. The studies were heterogeneous in terms of the interventions delivered, outcomes measured and timing of outcome assessment, therefore, we were unable to pool results across studies. Only four studies reported the primary outcome of interest, short-term overall improvement (any measure in which patients indicate the intensity of their complaints compared to baseline, for example, global rating of improvement, satisfaction with treatment, within three months post-treatment). However, of these, only three fully reported outcome data sufficient for inclusion in the review. One very low quality trial with 14 participants found that all participants receiving either neurodynamic mobilisation or carpal bone mobilisation and none in the no treatment group reported overall improvement (RR 15.00, 95% CI 1.02 to 220.92), though the precision of this effect estimate is very low. One low quality trial with 22 participants found that the chance of being 'satisfied' or 'very satisfied' with treatment was 24% higher for participants receiving instrument-assisted soft tissue mobilisation compared to standard soft tissue mobilisation (RR 1.24, 95% CI 0.89 to 1.75), though participants were not blinded and it was unclear if the allocation sequence was concealed. Another very low-quality trial with 26 participants found that more CTS-affected wrists receiving nerve gliding exercises plus splint plus activity modification had no pathologic finding on median and ulnar nerve distal sensory latency assessment at the end of treatment than wrists receiving splint plus activity modification alone (RR 1.26, 95% CI 0.69 to 2.30). However, a unit of analysis error occurred in this trial, as the correlation between wrists in participants with bilateral CTS was not accounted for. Only two studies measured adverse effects, so more data are required before any firm conclusions on the safety of exercise and mobilisation interventions can be made. In general, the results of secondary outcomes of the review (short- and long-term improvement in CTS symptoms, functional ability, health-related quality of life, neurophysiologic parameters, and the need for surgery) for most comparisons had 95% CIs which incorporated effects in either direction.
Authors' conclusions: There is limited and very low quality evidence of benefit for all of a diverse collection of exercise and mobilisation interventions for CTS. People with CTS who indicate a preference for exercise or mobilisation interventions should be informed of the limited evidence of effectiveness and safety of this intervention by their treatment provider. Until more high quality randomised controlled trials assessing the effectiveness and safety of various exercise and mobilisation interventions compared to other non-surgical interventions are undertaken, the decision to provide this type of non-surgical intervention to people with CTS should be based on the clinician's expertise in being able to deliver these treatments and patient's preferences.
Conflict of interest statement
None known.
Figures


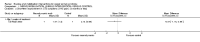






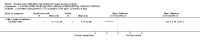



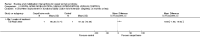


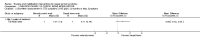




























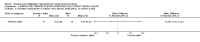
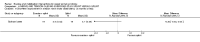
















































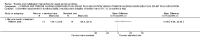
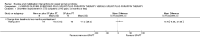
























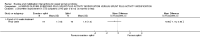


















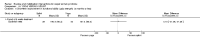
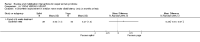
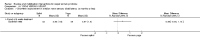
References
References to studies included in this review
Akalin 2002 {published and unpublished data}
-
- Akalin E, El O, Peker O, Senocak O, Tamci S, Gülbahar S, et al. Treatment of carpal tunnel syndrome with nerve and tendon gliding exercises. American Journal of Physical Medicine and Rehabilitation 2002;81(2):108‐13. [PUBMED: 11807347] - PubMed
Bahrami 2006 {published data only}
-
- Bahrami MH, Rayegani SM, Baghbani M, Bafghi MRB. The role of nerve and tendon gliding exercises in the conservative treatment of carpal tunnel syndrome. Journal of Medical Council of Islamic Republic of Iran 2006;24(1):5‐12.
Bardak 2009 {published data only}
-
- Bardak AN, Alp M, Erhan B, Paker N, Kaya B, Onal AE. Evaluation of the clinical efficacy of conservative treatment in the management of carpal tunnel syndrome. Advances in Therapy 2009;26(1):107‐16. [PUBMED: 19165436] - PubMed
Baysal 2006 {published data only}
-
- Baysal O, Altay Z, Ozcan C, Ertem K, Yologlu S, Kayhan A. Comparison of three conservative treatment protocols in carpal tunnel syndrome. International Journal of Clinical Practice 2006;60(7):820‐8. [PUBMED: 16704676] - PubMed
Bialosky 2009 {published and unpublished data}
Brininger 2007 {published data only}
-
- Brininger TL, Rogers JC, Holm MB, Baker NA, Li Z‐M, Goitz RJ. Efficacy of a fabricated customized splint and tendon and nerve gliding exercises for the treatment of carpal tunnel syndrome: a randomized controlled trial. Archives of Physical Medicine and Rehabilitation 2007;88(11):1429‐35. [PUBMED: 17964883] - PubMed
Burke 2007 {published data only}
-
- Burke J, Buchberger DJ, Carey‐Loghmani MT, Dougherty PE, Greco DS, Dishman JD. A pilot study comparing two manual therapy interventions for carpal tunnel syndrome. Journal of Manipulative and Physiological Therapeutics 2007;30(1):50‐61. [PUBMED: 17224356] - PubMed
Davis 1998 {published data only}
-
- Davis PT, Hulbert JR, Kassak KM, Meyer JJ. Comparative efficacy of conservative medical and chiropractic treatments for carpal tunnel syndrome: a randomized clinical trial. Journal of Manipulative and Physiological Therapeutics 1998;21(5):317‐26. [PUBMED: 9627862] - PubMed
Field 2004 {published data only}
-
- Field T, Diego M, Cullen C, Hartshorn K, Gruskin A, Hernandez‐Reif M, et al. Carpal tunnel syndrome symptoms are lessened following massage therapy. Journal of Bodywork and Movement Therapies 2004;8(1):9‐14. [EMBASE: 2004016426]
Garfinkel 1998 {published data only}
-
- Garfinkel MS, Singhal A, Katz WA, Allan DA, Reshetar R, Schumacher HR Jr. Yoga‐based intervention for carpal tunnel syndrome: a randomized trial. JAMA 1998;280(18):1601‐3. [PUBMED: 9820263] - PubMed
Heebner 2008 {published and unpublished data}
-
- Heebner ML, Roddey TS. The effects of neural mobilization in addition to standard care in persons with carpal tunnel syndrome from a community hospital. Journal of Hand Therapy 2008;21(3):229‐241. [PUBMED: 18652967] - PubMed
Horng 2011 {published data only}
-
- Horng YS, Hsieh SF, Lin MC, Wang JD. A comparative study on effectiveness of tendon versus nerve gliding exercises for carpal tunnel syndrome. Pain Medicine 2010;11(2):310‐1. [EMBASE: 70212068]
-
- Horng YS, Hsieh SF, Tu YK, Lin MC, Horn YS, Wang JD. The comparative effectiveness of tendon and nerve gliding exercises in patients with carpal tunnel syndrome: a randomized trial. American Journal of Physical Medicine and Rehabilitation 2011;90(6):435‐42. [PUBMED: 21430512] - PubMed
Janssen 2009 {published data only}
-
- Janssen RG, Schwartz DA, Velleman PF. A randomized controlled study of contrast baths on patients with carpal tunnel syndrome. Journal of Hand Therapy 2009;22(3):200‐8. [PUBMED: 19375278] - PubMed
Moraska 2008 {published and unpublished data}
-
- Moraska A, Chandler C, Edmiston‐Schaetzel A, Franklin G, Calenda EL, Enebo B. Comparison of a targeted and general massage protocol on strength, function, and symptoms associated with carpal tunnel syndrome: a randomized pilot study. Journal of Alternative and Complementary Medicine 2008;14(3):259‐67. [PUBMED: 18370581] - PubMed
Pinar 2005 {published and unpublished data}
-
- Pinar L, Enhos A, Ada S, Güngör N. Can we use nerve gliding exercises in women with carpal tunnel syndrome?. Advances in Therapy 2005;22(5):467‐75. [PUBMED: 16418156] - PubMed
Tal‐Akabi 2000 {published and unpublished data}
-
- Tal‐Akabi A, Rushton A. An investigation to compare the effectiveness of carpal bone mobilisation and neurodynamic mobilisation as methods of treatment for carpal tunnel syndrome. Manual Therapy 2000;5(4):214‐22. [PUBMED: 11052900] - PubMed
References to studies excluded from this review
Abbot 1999 {published data only}
-
- Abbot NC. Yoga‐based intervention for carpal tunnel syndrome: commentary. Focus on Alternative and Complementary Therapies 1999;4:81‐2.
Arinci Incel 2005 {published data only}
-
- Arinci Incel N, Sezgin M, Aksit C, Sahin G. Effect of gabapentin in carpal tunnel syndrome: a controlled clinical trial [Karpal tunnel sendromunda gabapentin tedavisinin etkinligi: karsilastirmali klinik calisma]. Journal of Rheumatology and Medical Rehabilitation 2005;16(4):224‐30. [EMBASE: 2007022406]
Bernaards 2006 {published data only}
Blankfield 2001 {published data only}
-
- Blankfield RP, Sulzmann C, Fradley LG, Tapolyai AA, Zyzanski SJ. Therapeutic touch in the treatment of carpal tunnel syndrome. Journal of the American Board of Family Practice 2001;14(5):335‐42. [PUBMED: 11572538] - PubMed
George 2006 {published data only}
Giattini 1999 {published data only}
-
- Giattini A, Logullo F, Morici D, Castellani G, Provinciali L. Physiotherapy reduced the recovery time after carpal tunnel syndrome. Neurorehabilitation and Neural Repair 1999;13(1):51.
Goldberg 2004 {published data only}
-
- Goldberg H. Massage decreases carpal tunnel syndrome pain. Focus on Alternative and Complementary Therapies 2004;9(2):141‐2.
Hains 2010 {published data only}
Nathan 2001 {published data only}
-
- Nathan PA, Wilcox A, Emerick PS, Meadows KD, McCormack AL. Effects of an aerobic exercise program on median nerve conduction and symptoms associated with carpal tunnel syndrome. Journal of Occupational and Environmental Medicine 2001;43(10):840‐3. [PUBMED: 11665452] - PubMed
Nathan 2002 {published data only}
-
- Nathan PA. Effects of an aerobic exercise program on median nerve conduction and symptoms associated with carpal tunnel syndrome. Journal of Occupational and Environmental Medicine 2002;44(4):303‐5. [PUBMED: 11977412] - PubMed
Omer 2003/2004 {published data only}
-
- Omer SR, Ozcan E, Karan A, Ketenci A. Musculoskeletal system disorders in computer users: effectiveness of training and exercise programs. Journal of Back and Musculoskeletal Rehabilitation 2003/2004;17(1):9‐13. [EMBASE: 2004195653]
Rozmaryn 1998 {published data only}
-
- Rozmaryn LM, Dovelle S, Rothman ER, Gorman K, Olvey KM, Bartko JJ. Nerve and tendon gliding exercises and the conservative management of carpal tunnel syndrome. Journal of Hand Therapy 1998;11(3):171‐9. [PUBMED: 9730093] - PubMed
Ruksen 2011 {published data only}
-
- Ruksen S, Oz B, Olmez N, Memis A. Comparison of clinical effectiveness of corticosteroid phonophoresis and local steroid injection treatment in carpal tunnel syndrome. Turkiye Fiziksel Tip ve Rehabilitasyon Dergisi 2011;57(3):119‐23.
Taspinar 2007 {published data only}
-
- Taşpinar Ş, Şahin F, Erçalik C, Kuran B, Barkut K, Çelik M, et al. Comparison of the efficacy of corticosteroid injection, night splint, and physiotherapy in diabetic carpal tunnel syndrome [Diyabetik karpal tünel sendromunda kortikosteroid enjeksiyonu, gece ateli ve fizik tedavinin etkinliğinin karşılaştırılması]. Turkish Journal of Physical Medicine and Rehabilitation 2007;53(2):54‐60.
Thomas 1993 {published data only}
-
- Thomas RE, Butterfield RK, Hool JN, Herrick RT. Effects of exercise on carpal tunnel syndrome symptoms. Applied Ergonomics 1993;24(2):101‐8. [EMBASE: 1993148379 ] - PubMed
Verhagen 2007 {published data only}
-
- Verhagen AP, Karels C, Bierma‐Zeinstra SMA, Feleus A, Dahaghin S, Burdorf A, et al. Exercise proves effective in a systematic review of work‐related complaints of the arm, neck, or shoulder. Journal of Clinical Epidemiology 2007;60(2):110‐7. [PUBMED: 17208116] - PubMed
Walker 2010 {published data only}
-
- Walker C. Automated manipulative device alleviates symptoms of carpal tunnel syndrome. Journal of Manual & Manipulative Therapy 2010;18(4):238.
References to studies awaiting assessment
Ashraf 2009 {published data only}
-
- Ashraf A, Moghtaderi AR, Yazdani AH, Mirshams S. Evaluation of effectiveness of local insulin injection in none insulin dependent diabetic patient with carpal tunnel syndrome. Electromyography and Clinical Neurophysiology 2009;49(4):161‐6. - PubMed
Avci 2004 {published data only}
-
- Avci S, Günaydin R, Öztura I. Comparison of effectiveness of splint and splint with physical therapy in carpal tunnel syndrome [Karpal tünel sendromunda atel ve atel ile birlikte fizik tedavinin etkinliğinin karşılaştırılması]. Türkiye Fiziksel Tip Ve Rehabilitasyon Dergisi 2004;50(2):22‐6.
El Miedany 2009 {published data only}
-
- Miedany Y, Gaafary M, Ahmed I, Mehanna A, Youssef S. Massage in carpal tunnel syndrome: a rational approach. Rheumatology 2009;48(Suppl 1):i95‐i96.
Maltese 2006 {published data only}
-
- Maltese V, Cataldo P, Cusumano C, Sanfilippo A, Letizia G. Therapeutic outcome assessment in the carpal tunnel syndrome: Therapies' confrontation. Acta Medica Mediterranea 2006;22(1):29‐32.
Shi 2006 {published data only}
-
- Shi YS, Fang W, Zhao XY. Control study on effect of pricking collateral blood therapy combined with massage on mild carpal tunnel syndrome. Zhongguo Zhong xi yi jie he za zhi Zhongguo Zhongxiyi jiehe zazhi [Chinese Journal of Integrated Traditional and Western Medicine] 2006;26(6):497‐99. - PubMed
Additional references
Atroshi 1999
-
- Atroshi I, Gummesson C, Johnsson R, Ornstein E, Ranstam J, Rosén I. Prevalence of carpal tunnel syndrome in a general population. JAMA 1999;282(2):153‐8. [PUBMED: 10411196] - PubMed
Bland 2005
-
- Bland JD. The relationship of obesity, age, and carpal tunnel syndrome: more complex than was thought?. Muscle & Nerve 2005;32(4):527‐32. - PubMed
Braun 1989
-
- Braun RM, Davidson K, Doehr S. Provocative testing in the diagnosis of dynamic carpal tunnel syndrome. Journal of Hand Surgery (American Volume) 1989;14(2 Pt 1):195‐7. [MEDLINE: ] - PubMed
Butler 1991
-
- Butler DS. Mobilization of the Nervous System. First Edition. Melbourne: Churchill Livingstone, 1991.
Charles 2009
-
- Charles J, Fahridin S, Britt H. Carpal tunnel syndrome. Australian Family Physician 2009;38(9):665. - PubMed
D'Arcy 2000
-
- D’Arcy CA, McGee S. The rational clinical examination. Does this patient have carpal tunnel syndrome?. JAMA 2000;283:3110‐7. - PubMed
Deeks 2011
-
- Deeks JJ, Higgins JPT, Altman DG. Analysing data and undertaking meta‐analyses. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011:Available from www.cochrane‐handbook.org.
Dwan 2008
Dwan 2011
Gelfman 2009
Gerritsen 2002
-
- Gerritsen AA, Krom MC, Struijs MA, Scholten RJ, Vet HC, Bouter LM. Conservative treatment options for carpal tunnel syndrome: a systematic review of randomised controlled trials. Journal of Neurology 2002;249(3):272‐80. - PubMed
Goodyear‐Smith 2004
Higgins 2002
-
- Higgins JPT, Thompson SG. Quantifying heterogeneity in meta‐analysis. Statistics in Medicine 2002;21(11):1539‐58. - PubMed
Higgins 2011a
-
- Higgins JPT, Green S (editors). Cochrane Handbook of Systematic Reviews of Interventions Version 5.1.0 [Updated March 2011]. The Cochrane Collaboration 2011. Available from www.cochrane‐handbook.org.
Higgins 2011b
-
- Higgins JPT, Altman DG, Sterne JAC. Assessing risk of bias in included studies. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011:Available from www.cochrane‐handbook.org.
Higgins 2011c
-
- Higgins JPT, Deeks JJ, Altman DG. Special topics in statistics. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011:Available from www.cochrane‐handbook.org.
Hopewell 2007
Huisstede 2010
-
- Huisstede BM, Hoogvliet P, Randsdorp MS, Glerum S, Middelkoop M, Koes BW. Carpal tunnel syndrome. Part 1: Effectiveness of nonsurgical treatments ‐ a systematic review. Archives of Physical Medicine and Rehabilitation 2010;91(7):981‐1004. - PubMed
Iyengar 1966
-
- Iyengar BKS. Light on yoga. London, England: Allen & Unwin, 1966.
Katz 1990
-
- Katz JN, Stirrat CR, Larson MG, Fossel AH, Eaton HM, Liang MH. A self‐administered hand symptom diagram for the diagnosis and epidemiologic study of carpal tunnel syndrome. Journal of Rheumatology 1990;17(11):1495‐8. [PUBMED: 2273490 ] - PubMed
Keith 2009
Kerwin 1996
-
- Kerwin G, Williams CS, Seiler JG 3rd. The pathophysiology of carpal tunnel syndrome. Hand Clinics 1996;12(2):243‐51. [PUBMED: 8724576] - PubMed
Kirkham 2010
-
- Kirkham JJ, Dwan KM, Altman DG, Gamble C, Dodd S, Smyth R, et al. The impact of outcome reporting bias in randomised controlled trials on a cohort of systematic reviews. BMJ 2010;340:c365. [PUBMED: 20156912] - PubMed
Levine 1993
-
- Levine DW, Simmons BP, Koris MJ, Daltroy LH, Hohl GG, Fossel AH, et al. A self‐administered questionnaire for the assessment of severity of symptoms and functional status in carpal tunnel syndrome. Journal of Bone and Joint Surgery 1993;75(11):1585‐92. [PUBMED: 8245050] - PubMed
Maitland 1991
-
- Maitland GD. Peripheral Manipulation. 3rd Edition. Oxford: Butterworth‐Heinemann, 1991.
Marshall 2007
McKeon 2008
-
- McKeon JMM, Yancosek KE. Neural gliding techniques for the treatment of carpal tunnel syndrome: a systematic review. Journal of Sport Rehabilitation 2008;17(3):324‐41. - PubMed
Muller 2004
-
- Muller M, Tsui D, Schnurr R, Biddulph‐Deisroth L, Hard J, MacDermid JC. Effectiveness of hand therapy interventions in primary management of carpal tunnel syndrome: a systematic review. Journal of Hand Therapy 2004;17(2):210‐28. - PubMed
O'Connor 2012
Odgaard‐Jensen 2011
Ono 2010
Page 2012
Phalen 1966
-
- Phalen GS. The carpal tunnel syndrome. Journal of Bone and Joint Surgery 1966;48A:211‐28. - PubMed
Piazzini 2007
-
- Piazzini DB, Aprile I, Ferrera PE, Bertolini C, Tonali P, Maggi L, et al. A systematic review of conservative treatment of carpal tunnel syndrome. Clinical Rehabilitation 2007;21(4):299‐314. - PubMed
Rempel 1998
Scholten 2007
Stallings 1997
-
- Stallings SP, Kasdan ML, Soergel TM, Corwin HM. A case‐control study of obesity as a risk factor for carpal tunnel syndrome in a population of 600 patients presenting for independent medical examination. The Journal of Hand Surgery 1997;22(2):211‐5. - PubMed
Sterne 2011
-
- Sterne JAC, Egger M, Moher D. Addressing reporting biases. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011:Available from www.cochrane‐handbook.org.
Szabo 1992
-
- Szabo RM, Madison M. Carpal tunnel syndrome. Orthopedic Clinics of North America 1992;23(1):103‐9. [PUBMED: 1729659] - PubMed
Szabo 1994
-
- Szabo RM, Steinberg DR. Nerve entrapment syndromes in the wrist. Journal of the American Academy of Orthopedic Surgeons 1994;2(2):115‐23. - PubMed
Totten 1991
-
- Totten PA, Hunter JM. Therapeutic techniques to enhance nerve gliding in thoracic outlet syndrome and carpal tunnel syndrome. Hand Clinics 1991;7:505‐20. - PubMed
Verdugo 2008
Werner 1994a
-
- Werner RA, Albers JW, Franzblau A, Armstrong TJ. The relationship between body mass index and the diagnosis of carpal tunnel syndrome. Muscle & Nerve 1994;17(6):632‐6. - PubMed
Werner 1994b
-
- Werner R, Bir C, Armstrong T. Reverse Phalen's maneuver as an aid in diagnosing carpal tunnel syndrome. Archives of Physical Medicine and Rehabilitation 1994;75(7):783‐6. - PubMed
References to other published versions of this review
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials

