Regulation of embryonic stem cell pluripotency by heat shock protein 90
- PMID: 22696450
- PMCID: PMC3665290
- DOI: 10.1002/stem.1143
Regulation of embryonic stem cell pluripotency by heat shock protein 90
Abstract
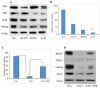
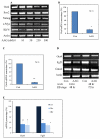
Deciphering the molecular basis of stem cell pluripotency is fundamental to the understanding of stem cell biology, early embryonic development, and to the clinical application of regenerative medicine. We report here that the molecular chaperone heat shock protein 90 (Hsp90) is essential for mouse embryonic stem cell (ESC) pluripotency through regulating multiple pluripotency factors, including Oct4, Nanog, and signal transducer and activator of transcription 3. Inhibition of Hsp90 by either 17-N-Allylamino-17-demethoxygeldanamycin or miRNA led to ESC differentiation. Overexpression of Hsp90β partially rescued the phenotype; in particular, the levels of Oct4 and Nanog were restored. Notably, Hsp90 associated with Oct4 and Nanog in the same cellular complex and protected them from degradation by the ubiquitin proteasome pathway, suggesting that Oct4 and Nanog are potential novel Hsp90 client proteins. In addition, Hsp90 inhibition reduced the mRNA level of Oct4, but not that of Nanog, indicating that Hsp90 participates in Oct4 mRNA processing or maturation. Hsp90 inhibition also increased expression of some protein markers for mesodermal lineages, implying that Hsp90 suppresses mesodermal differentiation from ESCs. These findings support a new role for Hsp90 in maintaining ESC pluripotency by sustaining the level of multiple pluripotency factors, particularly Oct4 and Nanog.
Copyright © 2012 AlphaMed Press.
Figures




References
-
- Evans MJ, Kaufman MH. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292:154–156. - PubMed
-
- Mountford P, Nichols J, Zevnik B, et al. Maintenance of pluripotential embryonicstem cells by stem cell selection. Reprod Fertil Dev. 1998;10:527–533. - PubMed
-
- Nichols J, Zevnik B, Anastassiadis K, et al. Formation of pluripotent stem cells in the mammalian embryo depends on the POU transcription factor Oct4. Cell. 1998;95:379–391. - PubMed
-
- Kim JB, Sebastiano V, Wu G, et al. Oct4-induced pluripotency in adult neural stem cells. Cell. 2009;136:411–419. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

