Traumatic brain injury in young rats leads to progressive behavioral deficits coincident with altered tissue properties in adulthood
- PMID: 22697253
- PMCID: PMC3408248
- DOI: 10.1089/neu.2011.1883
Traumatic brain injury in young rats leads to progressive behavioral deficits coincident with altered tissue properties in adulthood
Abstract
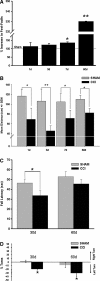
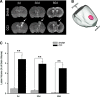
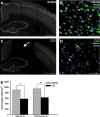
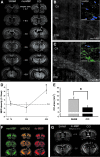
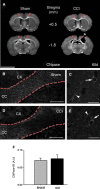
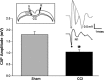
Traumatic brain injury (TBI) affects many infants and children, and results in enduring motor and cognitive impairments with accompanying changes in white matter tracts, yet few experimental studies in rodent juvenile models of TBI (jTBI) have examined the timeline and nature of these deficits, histologically and functionally. We used a single controlled cortical impact (CCI) injury to the parietal cortex of rats at post-natal day (P) 17 to evaluate behavioral alterations, injury volume, and morphological and molecular changes in gray and white matter, with accompanying measures of electrophysiological function. At 60 days post-injury (dpi), we found that jTBI animals displayed behavioral deficits in foot-fault and rotarod tests, along with a left turn bias throughout their early developmental stages and into adulthood. In addition, anxiety-like behaviors on the zero maze emerged in jTBI animals at 60 dpi. The final lesion constituted only ∼3% of brain volume, and morphological tissue changes were evaluated using MRI, as well as immunohistochemistry for neuronal nuclei (NeuN), myelin basic protein (MBP), neurofilament-200 (NF200), and oligodendrocytes (CNPase). White matter morphological changes were associated with a global increase in MBP immunostaining and reduced compound action potential amplitudes at 60 dpi. These results suggest that brain injury early in life can induce long-term white matter dysfunction, occurring in parallel with the delayed development and persistence of behavioral deficits, thus modeling clinical and longitudinal TBI observations.
Figures


References
-
- Adelson P.D. Dixon C.E. Kochanek P.M. Long-term dysfunction following diffuse traumatic brain injury in the immature rat. J. Neurotrauma. 2000;17:273–282. - PubMed
-
- Akiyama K. Ichinose S. Omori A. Sakurai Y. Asou H. Study of expression of myelin basic proteins (MBPs) in developing rat brain using a novel antibody reacting with four major isoforms of MBP. J. Neurosci. Res. 2002;68:19–28. - PubMed
-
- Badaut J. Ashwal S. Tone B. Regli L. Tian H.R. Obenaus A. Temporal and regional evolution of aquaporin-4 expression and magnetic resonance imaging in a rat pup model of neonatal stroke. Pediatr. Res. 2007;62:248–254. - PubMed
-
- Bloch J. Kaeser M. Sadeghi Y. Rouiller E.M. Redmond D.E., Jr. Brunet J.F. Doublecortin-positive cells in the adult primate cerebral cortex and possible role in brain plasticity and development. J. Comp. Neurol. 2011;519:775–789. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

