Fluoxetine induces vasodilatation of cerebral arterioles by co-modulating NO/muscarinic signalling
- PMID: 22697296
- PMCID: PMC4118242
- DOI: 10.1111/j.1582-4934.2012.01596.x
Fluoxetine induces vasodilatation of cerebral arterioles by co-modulating NO/muscarinic signalling
Abstract
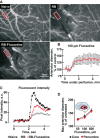
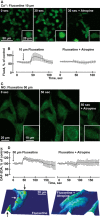
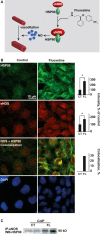
Ischaemic stroke patients treated with Selective Serotonin Reuptake Inhibitors (SSRI) show improved motor, cognitive and executive functions, but the underlying mechanism(s) are incompletely understood. Here, we report that cerebral arterioles in the rat brain superfused with therapeutically effective doses of the SSRI fluoxetine showed consistent, dose-dependent vasodilatation (by 1.2 to 1.6-fold), suppressible by muscarinic and nitric oxide synthase (NOS) antagonists [atropine, NG-nitro-l-arginine methyl ester (l-NAME)] but resistant to nicotinic and serotoninergic antagonists (mecamylamine, methylsergide). Fluoxetine administered 10-30 min. following experimental vascular photo-thrombosis increased arterial diameter (1.3-1.6), inducing partial, but lasting reperfusion of the ischaemic brain. In brain endothelial b.End.3 cells, fluoxetine induced rapid muscarinic receptor-dependent increases in intracellular [Ca(2+) ] and promoted albumin- and eNOS-dependent nitric oxide (NO) production and HSP90 interaction. In vitro, fluoxetine suppressed recombinant human acetylcholinesterase (rhAChE) activity only in the presence of albumin. That fluoxetine induces vasodilatation of cerebral arterioles suggests co-promotion of endothelial muscarinic and nitric oxide signalling, facilitated by albumin-dependent inhibition of serum AChE.
© 2012 The Authors Journal of Cellular and Molecular Medicine © 2012 Foundation for Cellular and Molecular Medicine/Blackwell Publishing Ltd.
Figures


References
-
- Shuaib A, Butcher K, Mohammad AA, et al. Collateral blood vessels in acute ischaemic stroke: a potential therapeutic target. Lancet Neurol. 2011;10:909–21. - PubMed
-
- Li WL, Cai HH, Wang B, et al. Chronic fluoxetine treatment improves ischaemia-induced spatial cognitive deficits through increasing hippocampal neurogenesis after stroke. J Neurosci Res. 2009;87:112–22. - PubMed
-
- Chollet F, Tardy J, Albucher JF, et al. Fluoxetine for motor recovery after acute ischaemic stroke (FLAME): a randomised placebo-controlled trial. Lancet Neurol. 2011;10:123–30. - PubMed
-
- Narushima K, Paradiso S, Moser DJ, et al. Effect of antidepressant therapy on executive function after stroke. Br J Psychiatry. 2007;190:260–5. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

