Forward genetics uncovers Transmembrane protein 107 as a novel factor required for ciliogenesis and Sonic hedgehog signaling
- PMID: 22698544
- PMCID: PMC3402655
- DOI: 10.1016/j.ydbio.2012.06.008
Forward genetics uncovers Transmembrane protein 107 as a novel factor required for ciliogenesis and Sonic hedgehog signaling
Abstract
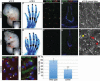
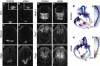
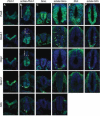
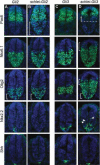
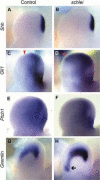
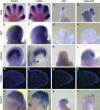
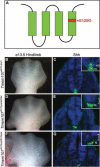
Cilia are dynamic organelles that are essential for a vast array of developmental patterning events, including left-right specification, skeletal formation, neural development, and organogenesis. Despite recent advances in understanding cilia form and function, many key ciliogenesis components have yet to be identified. By using a forward genetics approach, we isolated a novel mutant allele (schlei) of the mouse Transmembrane protein 107 (Tmem107) gene, which we show here is critical for cilia formation and embryonic patterning. Tmem107 is required for normal Sonic hedgehog (Shh) signaling in the neural tube and acts in combination with Gli2 and Gli3 to pattern ventral and intermediate neuronal cell types. schlei mutants also form extra digits, and we demonstrate that Tmem107 acts in the Shh pathway to determine digit number, but not identity, by regulating a subset of Shh target genes. Phenotypically, schlei mutants share several features with other cilia mutants; however, spatial restriction of mutant phenotypes and lack of left-right patterning defects in schlei animals suggest differential requirements for Tmem107 in cilia formation in distinct tissues. Also, in contrast to mutants with complete loss of cilia, schlei mutants retain some function of both Gli activator and repressor forms. Together, these studies identify a previously unknown regulator of ciliogenesis and provide insight into how ciliary factors affect Shh signaling and cilia biogenesis in distinct tissues.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures
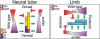
References
-
- Ansley SJ, Badano JL, Blacque OE, Hill J, Hoskins BE, Leitch CC, Kim JC, Ross AJ, Eichers ER, Teslovich TM, et al. Basal body dysfunction is a likely cause of pleiotropic Bardet-Biedl syndrome. Nature. 2003;425(6958):628–33. - PubMed
-
- Avidor-Reiss T, Maer AM, Koundakjian E, Polyanovsky A, Keil T, Subramaniam S, Zuker CS. Decoding cilia function: defining specialized genes required for compartmentalized cilia biogenesis. Cell. 2004;117(4):527–39. - PubMed
-
- Bai CB, Auerbach W, Lee JS, Stephen D, Joyner AL. Gli2, but not Gli1, is required for initial Shh signaling and ectopic activation of the Shh pathway. Development. 2002;129(20):4753–61. - PubMed
-
- Bai CB, Joyner AL. Gli1 can rescue the in vivo function of Gli2. Development. 2001;128(24):5161–72. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

