Tissue transglutaminase overexpression does not modify the disease phenotype of the R6/2 mouse model of Huntington's disease
- PMID: 22698685
- PMCID: PMC3418489
- DOI: 10.1016/j.expneurol.2012.05.015
Tissue transglutaminase overexpression does not modify the disease phenotype of the R6/2 mouse model of Huntington's disease
Abstract
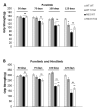
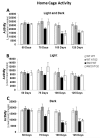
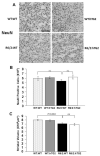
Huntington's disease (HD) is a devastating autosomal-dominant neurodegenerative disorder initiated by an abnormally expanded polyglutamine in the huntingtin protein. Determining the contribution of specific factors to the pathogenesis of HD should provide rational targets for therapeutic intervention. One suggested contributor is the type 2 transglutaminase (TG2), a multifunctional calcium dependent enzyme. A role for TG2 in HD has been suggested because a polypeptide-bound glutamine is a rate-limiting factor for a TG2-catalyzed reaction, and TG2 can cross-link mutant huntingtin in vitro. Further, TG2 is up regulated in brain areas affected in HD. The objective of this study was to further examine the contribution of TG2 as a potential modifier of HD pathogenesis and its validity as a therapeutic target in HD. In particular our goal was to determine whether an increase in TG2 level, as documented in human HD brains, modulates the well-characterized phenotype of the R6/2 HD mouse model. To accomplish this objective a genetic cross was performed between R6/2 mice and an established transgenic mouse line that constitutively expresses human TG2 (hTG2) under control of the prion promoter. Constitutive expression of hTG2 did not affect the onset and progression of the behavioral and neuropathological HD phenotype of R6/2 mice. We found no alterations in body weight changes, rotarod performances, grip strength, overall activity, and no significant effect on the neuropathological features of R6/2 mice. Overall the results of this study suggest that an increase in hTG2 expression does not significantly modify the pathology of HD.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures








Similar articles
-
Genetic deletion of transglutaminase 2 does not rescue the phenotypic deficits observed in R6/2 and zQ175 mouse models of Huntington's disease.PLoS One. 2014 Jun 23;9(6):e99520. doi: 10.1371/journal.pone.0099520. eCollection 2014. PLoS One. 2014. PMID: 24955833 Free PMC article.
-
Tissue transglutaminase contributes to disease progression in the R6/2 Huntington's disease mouse model via aggregate-independent mechanisms.J Neurochem. 2005 Jan;92(1):83-92. doi: 10.1111/j.1471-4159.2004.02839.x. J Neurochem. 2005. PMID: 15606898
-
Type 2 transglutaminase differentially modulates striatal cell death in the presence of wild type or mutant huntingtin.J Neurochem. 2007 Jul;102(1):25-36. doi: 10.1111/j.1471-4159.2007.04491.x. Epub 2007 Mar 30. J Neurochem. 2007. PMID: 17403029
-
Type 2 transglutaminase in Huntington's disease: a double-edged sword with clinical potential.J Intern Med. 2010 Nov;268(5):419-31. doi: 10.1111/j.1365-2796.2010.02275.x. J Intern Med. 2010. PMID: 20964734 Free PMC article. Review.
-
The use of the R6 transgenic mouse models of Huntington's disease in attempts to develop novel therapeutic strategies.NeuroRx. 2005 Jul;2(3):447-64. doi: 10.1602/neurorx.2.3.447. NeuroRx. 2005. PMID: 16389308 Free PMC article. Review.
Cited by
-
Transglutaminase 6 Is Colocalized and Interacts with Mutant Huntingtin in Huntington Disease Rodent Animal Models.Int J Mol Sci. 2021 Aug 18;22(16):8914. doi: 10.3390/ijms22168914. Int J Mol Sci. 2021. PMID: 34445621 Free PMC article.
-
SAR Development of Lysine-Based Irreversible Inhibitors of Transglutaminase 2 for Huntington's Disease.ACS Med Chem Lett. 2012 Oct 4;3(12):1024-8. doi: 10.1021/ml300241m. eCollection 2012 Dec 13. ACS Med Chem Lett. 2012. PMID: 24900424 Free PMC article.
-
Allelic series of Huntington's disease knock-in mice reveals expression discorrelates.Hum Mol Genet. 2016 Apr 15;25(8):1619-36. doi: 10.1093/hmg/ddw040. Epub 2016 Feb 14. Hum Mol Genet. 2016. PMID: 26908599 Free PMC article.
-
Cooperation of cell adhesion and autophagy in the brain: Functional roles in development and neurodegenerative disease.Matrix Biol Plus. 2021 Oct 23;12:100089. doi: 10.1016/j.mbplus.2021.100089. eCollection 2021 Dec. Matrix Biol Plus. 2021. PMID: 34786551 Free PMC article. Review.
-
The protective role of exercise against age-related neurodegeneration.Ageing Res Rev. 2022 Feb;74:101543. doi: 10.1016/j.arr.2021.101543. Epub 2021 Dec 17. Ageing Res Rev. 2022. PMID: 34923167 Free PMC article. Review.
References
-
- Bailey CD, Johnson GV. Tissue transglutaminase contributes to disease progression in the R6/2 Huntington’s disease mouse model via aggregate-independent mechanisms. J Neurochem. 2005;92:83–92. - PubMed
-
- Bailey CD, Johnson GV. The protective effects of cystamine in the R6/2 Huntington’s disease mouse involve mechanisms other than the inhibition of tissue transglutaminase. Neurobiol Aging. 2006;27:871–879. - PubMed
-
- Beal MF. Huntington’s disease, energy, and excitotoxicity. Neurobiol Aging. 1994;15:275–276. - PubMed
-
- Beal MF. Mitochondria, free radicals, and neurodegeneration. Curr Opin Neurobiol. 1996;6:661–666. - PubMed
-
- Bezprozvanny I, Hayden MR. Deranged neuronal calcium signaling and Huntington disease. Biochemical and biophysical research communications. 2004;322:1310–1317. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

