Solid formulation of cell-penetrating peptide nanocomplexes with siRNA and their stability in simulated gastric conditions
- PMID: 22698942
- PMCID: PMC7126485
- DOI: 10.1016/j.jconrel.2012.06.006
Solid formulation of cell-penetrating peptide nanocomplexes with siRNA and their stability in simulated gastric conditions
Abstract
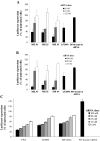
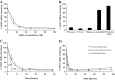
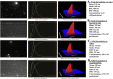
Cell-penetrating peptides (CPPs) are short cationic peptides that have been extensively studied as drug delivery vehicles for proteins, nucleic acids and nanoparticles. However, the formulation of CPP-based therapeutics into different pharmaceutical formulations and their stability in relevant biological environments have not been given the same attention. Here, we show that a newly developed CPP, PepFect 14 (PF14), forms non-covalent nanocomplexes with short interfering RNA (siRNA), which are able to elicit efficient RNA-interference (RNAi) response in different cell-lines. RNAi effect is obtained at low siRNA doses with a unique kinetic profile. Furthermore, the solid dispersion technique is utilized to formulate PF14/siRNA nanocomplexes into solid formulations that are as active as the freshly prepared nanocomplexes in solution. Importantly, the nanocomplexes are stable and active in mediating RNAi response after incubation with simulated gastric fluid (SGF) that is highly acidic. These results demonstrate the activity of PF14 in delivering and protecting siRNA in different pharmaceutical forms and biological environments.
Copyright © 2012 Elsevier B.V. All rights reserved.
Figures


References
-
- Ezzat K., El Andaloussi S., Abdo R., Langel Ü. Peptide-based matrices as drug delivery vehicles. Curr. Pharm. Des. 2010;16:1167–1178. - PubMed
-
- Verdurmen W.P., Brock R. Biological responses towards cationic peptides and drug carriers. Trends Pharmacol. Sci. 2011;32:116–124. - PubMed
-
- Deshayes S., Morris M., Heitz F., Divita G. Delivery of proteins and nucleic acids using a non-covalent peptide-based strategy. Adv. Drug Deliv. Rev. 2008;60:537–547. - PubMed
-
- Mäe M., El Andaloussi S., Lehto T., Langel Ü. Chemically modified cell-penetrating peptides for the delivery of nucleic acids. Expert Opin. Drug Deliv. 2009;6:1195–1205. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

