Production, characterization, and pharmacokinetic properties of antibodies with N-linked mannose-5 glycans
- PMID: 22699308
- PMCID: PMC3499342
- DOI: 10.4161/mabs.20737
Production, characterization, and pharmacokinetic properties of antibodies with N-linked mannose-5 glycans
Abstract
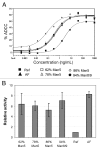
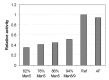
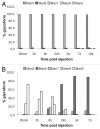
The effector functions of therapeutic antibodies are strongly affected by the specific glycans added to the Fc domain during post-translational processing. Antibodies bearing high levels of N-linked mannose-5 glycan (Man5) have been reported to exhibit enhanced antibody-dependent cell-mediated cytotoxicity (ADCC) compared with antibodies with fucosylated complex or hybrid glycans. To better understand the relationship between antibodies with high levels of Man5 and their biological activity in vivo, we developed an approach to generate substantially homogeneous antibodies bearing the Man5 glycoform. A mannosidase inhibitor, kifunensine, was first incorporated in the cell culture process to generate antibodies with a distribution of high mannose glycoforms. Antibodies were then purified and treated with a mannosidase for trimming to Man5 in vitro. This 2-step approach can consistently generate antibodies with > 99% Man5 glycan. Antibodies bearing varying levels of Man5 were studied to compare ADCC and Fcγ receptor binding, and they showed enhanced ADCC activity and increased binding affinity to the FcγRIIIA. In addition, the clearance rate of antibodies bearing Man8/9 and Man5 glycans was determined in a pharmacokinetics study in mice. When compared with historical data, the antibodies bearing the high mannose glycoform exhibited faster clearance rate compared with antibodies bearing the fucosylated complex glycoform, while the pharmacokinetic properties of antibodies with Man8/9 and Man5 glycoforms appeared similar. In addition, we identified the presence of a mannosidase in mouse serum that converted most Man8/9 to Man6 after 24 h.
Figures






Similar articles
-
Glycoform-resolved pharmacokinetic studies in a rat model employing glycoengineered variants of a therapeutic monoclonal antibody.MAbs. 2021 Jan-Dec;13(1):1865596. doi: 10.1080/19420862.2020.1865596. MAbs. 2021. PMID: 33382957 Free PMC article.
-
Comparison of biological activity among nonfucosylated therapeutic IgG1 antibodies with three different N-linked Fc oligosaccharides: the high-mannose, hybrid, and complex types.Glycobiology. 2007 Jan;17(1):104-18. doi: 10.1093/glycob/cwl057. Epub 2006 Sep 29. Glycobiology. 2007. PMID: 17012310
-
Glycoengineered Monoclonal Antibodies with Homogeneous Glycan (M3, G0, G2, and A2) Using a Chemoenzymatic Approach Have Different Affinities for FcγRIIIa and Variable Antibody-Dependent Cellular Cytotoxicity Activities.PLoS One. 2015 Jul 22;10(7):e0132848. doi: 10.1371/journal.pone.0132848. eCollection 2015. PLoS One. 2015. PMID: 26200113 Free PMC article.
-
The "less-is-more" in therapeutic antibodies: Afucosylated anti-cancer antibodies with enhanced antibody-dependent cellular cytotoxicity.MAbs. 2018 Jul;10(5):693-711. doi: 10.1080/19420862.2018.1466767. MAbs. 2018. PMID: 29733746 Free PMC article. Review.
-
The importance of IgG glycosylation-What did we learn after analyzing over 100,000 individuals.Immunol Rev. 2024 Nov;328(1):143-170. doi: 10.1111/imr.13407. Epub 2024 Oct 4. Immunol Rev. 2024. PMID: 39364834 Free PMC article. Review.
Cited by
-
Evaluation of Antibody Properties and Clinically Relevant Immunogenicity, Anaphylaxis, and Hypersensitivity Reactions in Two Phase III Trials of Tralokinumab in Severe, Uncontrolled Asthma.Drug Saf. 2019 Jun;42(6):769-784. doi: 10.1007/s40264-018-00788-w. Drug Saf. 2019. PMID: 30649752 Free PMC article. Clinical Trial.
-
Simultaneous Monitoring of Monoclonal Antibody Variants by Strong Cation-Exchange Chromatography Hyphenated to Mass Spectrometry to Assess Quality Attributes of Rituximab-Based Biotherapeutics.Int J Mol Sci. 2021 Aug 23;22(16):9072. doi: 10.3390/ijms22169072. Int J Mol Sci. 2021. PMID: 34445776 Free PMC article.
-
Regulatory considerations in the design, development and quality of monoclonal antibodies and related products for the diagnosis and treatment of cancer.Front Oncol. 2024 Apr 30;14:1379738. doi: 10.3389/fonc.2024.1379738. eCollection 2024. Front Oncol. 2024. PMID: 38746685 Free PMC article. Review.
-
Rapid Antibody Glycoengineering in CHO Cells Via RNA Interference and CGE-LIF N-Glycomics.Methods Mol Biol. 2022;2370:147-167. doi: 10.1007/978-1-0716-1685-7_7. Methods Mol Biol. 2022. PMID: 34611868
-
"Small is beautiful" - Examining reliable determination of low-abundant therapeutic antibody glycovariants.J Pharm Anal. 2024 Oct;14(10):100982. doi: 10.1016/j.jpha.2024.100982. Epub 2024 Apr 26. J Pharm Anal. 2024. PMID: 39850237 Free PMC article.
References
-
- Shields RL, Namenuk AK, Hong K, Meng YG, Rae J, Briggs JB, et al. High resolution mapping of the binding site on human IgG1 for Fc γ RI, Fc γ RII, Fc γ RIII, and FcRn and design of IgG1 variants with improved binding to the Fc γ R. J Biol Chem. 2001;276:6591–604. doi: 10.1074/jbc.M009483200. - DOI - PubMed
-
- Ferrara C, Grau S, Jäger C, Sondermann P, Brünker P, Waldhauer I, et al. Unique carbohydrate-carbohydrate interactions are required for high affinity binding between FcgammaRIII and antibodies lacking core fucose. Proc Natl Acad Sci U S A. 2011;108:12669–74. doi: 10.1073/pnas.1108455108. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
