Regulation of the metastatic cell phenotype by sialylated glycans
- PMID: 22699311
- PMCID: PMC4079276
- DOI: 10.1007/s10555-012-9359-7
Regulation of the metastatic cell phenotype by sialylated glycans
Abstract
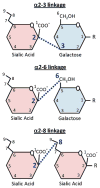
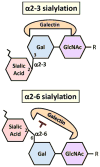
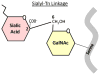
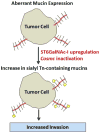
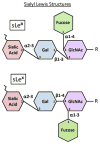
Tumor cells exhibit striking changes in cell surface glycosylation as a consequence of dysregulated glycosyltransferases and glycosidases. In particular, an increase in the expression of certain sialylated glycans is a prominent feature of many transformed cells. Altered sialylation has long been associated with metastatic cell behaviors including invasion and enhanced cell survival; however, there is limited information regarding the molecular details of how distinct sialylated structures or sialylated carrier proteins regulate cell signaling to control responses such as adhesion/migration or resistance to specific apoptotic pathways. The goal of this review is to highlight selected examples of sialylated glycans for which there is some knowledge of molecular mechanisms linking aberrant sialylation to critical processes involved in metastasis.
Figures




References
-
- Lloyd KO, Old LJ. Human monoclonal antibodies to glycolipids and other carbohydrate antigens: dissection of the humoral immune response in cancer patients. Cancer Research. 1989;49:3445–3451. - PubMed
-
- Sell S. Cancer-associated carbohydrates identified by monoclonal antibodies. Human Pathology. 1990;21:1003–1019. - PubMed
-
- Varki A, Kannagi R, Toole BP. Glycosylation changes in cancer. In: Varki A, Cummings RD, Esko JD, Freeze HH, Stanley P, Bertozzi CR, Hart GW, Etzler ME, editors. Essentials of Glycobiology. 2. New York: Cold Spring Harbor Laboratory Press; 2009. pp. 580–670. - PubMed
-
- Hakomori S. Aberrant glycosylation in tumors and tumor-associated carbohydrate antigens. Advances in Cancer Research. 1989;52:257–331. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

