Hypoxia-inducible factor 1 transcriptional activity in endothelial cells is required for acute phase cardioprotection induced by ischemic preconditioning
- PMID: 22699503
- PMCID: PMC3387090
- DOI: 10.1073/pnas.1208314109
Hypoxia-inducible factor 1 transcriptional activity in endothelial cells is required for acute phase cardioprotection induced by ischemic preconditioning
Abstract
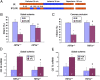
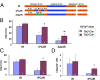
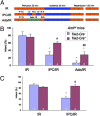
Infarction occurs when myocardial perfusion is interrupted for prolonged periods of time. Short episodes of ischemia and reperfusion protect against tissue injury when the heart is subjected to a subsequent prolonged ischemic episode, a phenomenon known as ischemic preconditioning (IPC). Hypoxia-inducible factor 1 (HIF-1) is a transcription factor that mediates adaptive responses to hypoxia/ischemia and is required for IPC. In this study, we performed a cellular and molecular characterization of the role of HIF-1 in IPC. We analyzed mice with knockout of HIF-1α or HIF-1β in Tie2(+) lineage cells, which include bone marrow (BM) and vascular endothelial cells, compared with control littermates. Hearts were subjected to 30 min of ischemia and 120 min of reperfusion, either as ex vivo Langendorff preparations or by in situ occlusion of the left anterior descending artery. The IPC stimulus consisted of two cycles of 5-min ischemia and 5-min reperfusion. Mice lacking HIF-1α or HIF-1β in Tie2(+) lineage cells showed complete absence of protection induced by IPC, whereas significant protection was induced by adenosine infusion. Treatment of mice with a HIF-1 inhibitor (digoxin or acriflavine) 4 h before Langendorff perfusion resulted in loss of IPC, as did administration of acriflavine directly into the perfusate immediately before IPC. We conclude that HIF-1 activity in endothelial cells is required for acute IPC. Expression and dimerization of the HIF-1α and HIF-1β subunits is required, suggesting that the heterodimer is functioning as a transcriptional activator, despite the acute nature of the response.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Hypoxia-inducible factor 1 is required for remote ischemic preconditioning of the heart.Proc Natl Acad Sci U S A. 2013 Oct 22;110(43):17462-7. doi: 10.1073/pnas.1317158110. Epub 2013 Oct 7. Proc Natl Acad Sci U S A. 2013. PMID: 24101519 Free PMC article.
-
Role of hypoxia inducible factor-1α in remote limb ischemic preconditioning.J Mol Cell Cardiol. 2013 Dec;65:98-104. doi: 10.1016/j.yjmcc.2013.10.001. Epub 2013 Oct 17. J Mol Cell Cardiol. 2013. PMID: 24140799
-
Roles of HIF-1α, VEGF, and NF-κB in Ischemic Preconditioning-Mediated Neuroprotection of Hippocampal CA1 Pyramidal Neurons Against a Subsequent Transient Cerebral Ischemia.Mol Neurobiol. 2017 Nov;54(9):6984-6998. doi: 10.1007/s12035-016-0219-2. Epub 2016 Oct 26. Mol Neurobiol. 2017. PMID: 27785755
-
Novel perspectives on the PHD-HIF oxygen sensing pathway in cardioprotection mediated by IPC and RIPC.Front Physiol. 2015 May 20;6:137. doi: 10.3389/fphys.2015.00137. eCollection 2015. Front Physiol. 2015. PMID: 26042040 Free PMC article. Review.
-
Keeping the engine primed: HIF factors as key regulators of cardiac metabolism and angiogenesis during ischemia.J Mol Med (Berl). 2007 Dec;85(12):1309-15. doi: 10.1007/s00109-007-0279-x. Epub 2007 Nov 20. J Mol Med (Berl). 2007. PMID: 18026917 Review.
Cited by
-
Three-Biomarker Joint Strategy for Early and Accurate Diagnosis of Acute Myocardial Infarction via a Multiplex Electrochemiluminescence Immunoarray Coupled with Robust Machine Learning.Chem Biomed Imaging. 2023 Apr 28;1(2):179-185. doi: 10.1021/cbmi.3c00035. eCollection 2023 May 22. Chem Biomed Imaging. 2023. PMID: 39474620 Free PMC article.
-
Infarct size-limiting effect of epoxyeicosatrienoic acid analog EET-B is mediated by hypoxia-inducible factor-1α via downregulation of prolyl hydroxylase 3.Am J Physiol Heart Circ Physiol. 2018 Nov 1;315(5):H1148-H1158. doi: 10.1152/ajpheart.00726.2017. Epub 2018 Aug 3. Am J Physiol Heart Circ Physiol. 2018. PMID: 30074840 Free PMC article.
-
Hypoxia Inducible Factors as Central Players in the Pathogenesis and Pathophysiology of Cardiovascular Diseases.Front Cardiovasc Med. 2021 Aug 10;8:709509. doi: 10.3389/fcvm.2021.709509. eCollection 2021. Front Cardiovasc Med. 2021. PMID: 34447792 Free PMC article. Review.
-
Kidney injury is independent of endothelial HIF-1α.J Mol Med (Berl). 2015 Aug;93(8):891-904. doi: 10.1007/s00109-015-1264-4. Epub 2015 Mar 11. J Mol Med (Berl). 2015. PMID: 25754172 Free PMC article.
-
Comparative Analysis of Heart Regeneration: Searching for the Key to Heal the Heart-Part II: Molecular Mechanisms of Cardiac Regeneration.J Cardiovasc Dev Dis. 2023 Aug 22;10(9):357. doi: 10.3390/jcdd10090357. J Cardiovasc Dev Dis. 2023. PMID: 37754786 Free PMC article. Review.
References
-
- Lopaschuk GD, Kelly DP. Signalling in cardiac metabolism. Cardiovasc Res. 2008;79:205–207. - PubMed
-
- Nallamothu BK, Bradley EH, Krumholz HM. Time to treatment in primary percutaneous coronary intervention. N Engl J Med. 2007;357:1631–1638. - PubMed
-
- Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: A delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124–1136. - PubMed
-
- Kuzuya T, et al. Delayed effects of sublethal ischemia on the acquisition of tolerance to ischemia. Circ Res. 1993;72:1293–1299. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

