Notch ligand delta-like 4 blockade attenuates atherosclerosis and metabolic disorders
- PMID: 22699504
- PMCID: PMC3390871
- DOI: 10.1073/pnas.1116889109
Notch ligand delta-like 4 blockade attenuates atherosclerosis and metabolic disorders
Abstract
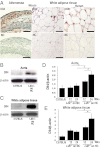
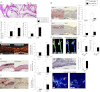
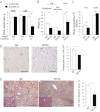
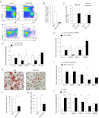
Atherosclerosis and insulin resistance are major components of the cardiometabolic syndrome, a global health threat associated with a systemic inflammatory state. Notch signaling regulates tissue development and participates in innate and adaptive immunity in adults. The role of Notch signaling in cardiometabolic inflammation, however, remains obscure. We noted that a high-fat, high-cholesterol diet increased expression of the Notch ligand Delta-like 4 (Dll4) in atheromata and fat tissue in LDL-receptor-deficient mice. Blockade of Dll4-Notch signaling using neutralizing anti-Dll4 antibody attenuated the development of atherosclerosis, diminished plaque calcification, improved insulin resistance, and decreased fat accumulation. These changes were accompanied by decreased macrophage accumulation, diminished expression of monocyte chemoattractant protein-1 (MCP-1), and lower levels of nuclear factor-κB (NF-κB) activation. In vitro cell culture experiments revealed that Dll4-mediated Notch signaling increases MCP-1 expression via NF-κB, providing a possible mechanism for in vivo effects. Furthermore, Dll4 skewed macrophages toward a proinflammatory phenotype ("M1"). These results suggest that Dll4-Notch signaling plays a central role in the shared mechanism for the pathogenesis of cardiometabolic disorders.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet. 2005;365:1415–1428. - PubMed
-
- Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860–867. - PubMed
-
- Artavanis-Tsakonas S, Rand MD, Lake RJ. Notch signaling: Cell fate control and signal integration in development. Science. 1999;284:770–776. - PubMed
-
- Radtke F, Fasnacht N, Macdonald HR. Notch signaling in the immune system. Immunity. 2010;32:14–27. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

