Peroxisome proliferator-activated receptor-γ as a therapeutic target for hepatic fibrosis: from bench to bedside
- PMID: 22699820
- PMCID: PMC11113701
- DOI: 10.1007/s00018-012-1046-x
Peroxisome proliferator-activated receptor-γ as a therapeutic target for hepatic fibrosis: from bench to bedside
Abstract
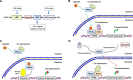
Hepatic fibrosis is a dynamic chronic liver disease occurring as a consequence of wound-healing responses to various hepatic injuries. This disorder is one of primary predictors for liver-associated morbidity and mortality worldwide. To date, no pharmacological agent has been approved for hepatic fibrosis or could be recommended for routine use in clinical context. Cellular and molecular understanding of hepatic fibrosis has revealed that peroxisome proliferator-activated receptor-γ (PPARγ), the functioning receptor for antidiabetic thiazolidinediones, plays a pivotal role in the pathobiology of hepatic stellate cells (HSCs), whose activation is the central event in the pathogenesis of hepatic fibrosis. Activation of PPARγ inhibits HSC collagen production and modulates HSC adipogenic phenotype at transcriptional and epigenetic levels. These molecular insights indicate PPARγ as a promising drug target for antifibrotic chemotherapy. Intensive animal studies have demonstrated that stimulation of PPARγ regulatory system through gene therapy approaches and PPARγ ligands has therapeutic promise for hepatic fibrosis induced by a variety of etiologies. At the same time, thiazolidinedione agents have been investigated for their clinical benefits primarily in patients with nonalcoholic steatohepatitis, a common metabolic liver disorder with high potential to progress to fibrosis and liver-related death. Although some studies have shown initial promise, none has established long-term efficacy in well-controlled randomized clinical trials. This comprehensive review covers the 10-year discoveries of the molecular basis for PPARγ regulation of HSC pathophysiology and then focuses on the animal investigations and clinical trials of various therapeutic modalities targeting PPARγ for hepatic fibrosis.
Figures
References
-
- Hernandez-Gea V, Friedman SL. Pathogenesis of liver fibrosis. Annu Rev Pathol. 2011;6:425–456. - PubMed
-
- Thabut D, Shah V. Intrahepatic angiogenesis and sinusoidal remodeling in chronic liver disease: new targets for the treatment of portal hypertension? J Hepatol. 2010;53(5):976–980. - PubMed
-
- Lim YS, Kim WR. The global impact of hepatic fibrosis and end-stage liver disease. Clin Liver Dis. 2008;12(4):733–746. - PubMed
-
- Anty R, Lemoine M. Liver fibrogenesis and metabolic factors. Clin Res Hepatol Gastroenterol. 2011;35(Suppl 1):S10–S20. - PubMed
-
- Romero-Gomez M, Del Mar Viloria M, Andrade RJ, Salmeron J, Diago M, Fernandez-Rodriguez CM, Corpas R, Cruz M, Grande L, Vazquez L, Munoz-De-Rueda P, Lopez-Serrano P, Gila A, Gutierrez ML, Perez C, Ruiz-Extremera A, Suarez E, Castillo J. Insulin resistance impairs sustained response rate to peginterferon plus ribavirin in chronic hepatitis C patients. Gastroenterology. 2005;128(3):636–641. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

