Ion channels and transporters in lymphocyte function and immunity
- PMID: 22699833
- PMCID: PMC3670817
- DOI: 10.1038/nri3233
Ion channels and transporters in lymphocyte function and immunity
Abstract
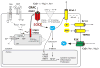
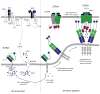
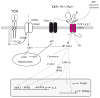
Lymphocyte function is regulated by a network of ion channels and transporters in the plasma membrane of B and T cells. These proteins modulate the cytoplasmic concentrations of diverse cations, such as calcium, magnesium and zinc ions, which function as second messengers to regulate crucial lymphocyte effector functions, including cytokine production, differentiation and cytotoxicity. The repertoire of ion-conducting proteins includes calcium release-activated calcium (CRAC) channels, P2X receptors, transient receptor potential (TRP) channels, potassium channels, chloride channels and magnesium and zinc transporters. This Review discusses the roles of ion conduction pathways in lymphocyte function and immunity.
Conflict of interest statement
Figures


References
-
- Lewis RS. Calcium signaling mechanisms in T lymphocytes. Annu Rev Immunol. 2001;19:497–521. - PubMed
-
- Parekh AB, Putney JW. Store-operated calcium channels. Physiol Rev. 2005;85:757–810. - PubMed
-
- Hoth M, Penner R. Depletion of intracellular calcium stores activates a calcium current in mast cells. Nature. 1992;355:353–356. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

