Focal hepatic lesions in Gd-EOB-DTPA enhanced MRI: the atlas
- PMID: 22700119
- PMCID: PMC3443279
- DOI: 10.1007/s13244-012-0179-7
Focal hepatic lesions in Gd-EOB-DTPA enhanced MRI: the atlas
Abstract
Objective: This article reviews the different technical aspects and pitfalls of gadolinium (Gd)-ethoxibenzyl (EOB)-diethylenetriamine pentaacetic acid (DTPA) and the advantages of the hepatocellular phase (HCP) and defines its specific imaging features of liver lesions.
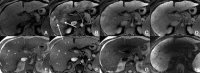
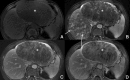
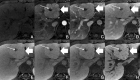
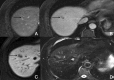
Background: Gd-EOB-DTPA is a contrast agent with combined properties of a conventional non-specific extracellular and a hepatocyte-specific contrast agent. Benign cirrhosis-associated nodules are characterised by isointensity in dynamic imaging and the HCP. Hepatocellular carcinomas (HCCs) usually show hyperenhancement in the arterial phase, with washout in the portal vein pressure (PVP) and hypointensity in the HCP. Among other characteristic findings, we have the mosaic pattern, a capsule, the "nodule-in-nodule" appearance and the satellite nodules. The fibrolamellar HCC is a large enhancing heterogeneous lesion, on a non-cirrhotic liver, with a hypointense scar in every sequence. THIDs (transient hepatic intensity differences) are perfusional alterations, characterised by hyperintensity in the arterial phase, with no alterations in the rest of the sequences including the HCP. Adenoma and focal nodular hyperplasia (FNH) are lesions, occurring more frequently in young women, with brisk arterial enhancement, differentiated by the scar and the uptake of Gd-EOB-DTPA in the HCP. Focal eosinophilic infiltration is a difficult diagnosis, with characteristics such as a non-spherical shape and irregular borders suggesting it. Besides these lesions, in which Gd-EOB-DTPA has a clear advantage, there are a few where the specificities of this agent can be troublesome: haemangiomas, focal fat/sparing, foreign body reaction, cholangiocarcinoma and metastases.
Conclusion: Gd-EOB-DTPA is comparable to extracellular agents, and uptake by functioning hepatocytes in the delayed phase provides additional information that further improves detection and characterisation of many hepatic lesions.
Main messages: Gd-EOB-DTPA offers advantages for the imaging of many liver lesions including HCC, fibrolamellar HCC, FNH and adenoma. • The properties of Gd-EOB-DTPA can pose problems when dealing with haemangiomas, cholangiocarcinoma and metastases among others. • The uptake of Gd-EOB-DTPA by functioning hepatocytes in the delayed phase provides additional information that further improves detection and characterisation of many hepatic lesions.
Figures


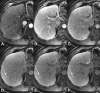
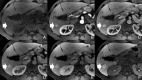
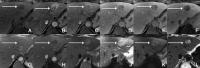
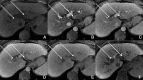




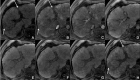
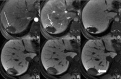
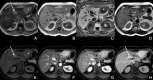




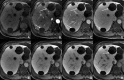

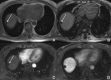



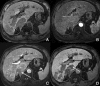
References
-
- Tsuboyama T, Onishi H, Kim T, Akita H, Hori M, Tatsumi M, Nakamoto A, Nagano H, Matsuura N, Wakasa K, Tomoda K. Hepatocellular carcinoma: hepatocyte-selective enhancement at gadoxetic acid–enhanced MR imaging—correlation with expression of sinusoidal and canalicular transporters and bile accumulation. Radiology. 2010;255(3):824–833. doi: 10.1148/radiol.10091557. - DOI - PubMed
-
- Kitao A, Zen Y, Matsui O, Gabata T, Kobayashi S, Koda W, Kozaka K, Yoneda N, Yamashita T, Kaneko S, Nakanuma Y. Hepatocellular carcinoma: signal intensity at gadoxetic acid– enhanced MR imaging—correlation with molecular transporters and histopathologic features. Radiology. 2010;256(3):817–826. doi: 10.1148/radiol.10092214. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

