Catalytic dynamic kinetic resolutions with N-heterocyclic carbenes: asymmetric synthesis of highly substituted β-lactones
- PMID: 22700327
- PMCID: PMC3489928
- DOI: 10.1002/anie.201203382
Catalytic dynamic kinetic resolutions with N-heterocyclic carbenes: asymmetric synthesis of highly substituted β-lactones
Erratum in
- Angew Chem Int Ed Engl. 2013 May 10;52(20):5203
Abstract
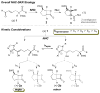
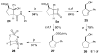
New DKR type: An N-heterocyclic carbene (NHC)-catalyzed dynamic kinetic resolution of racemic α-substituted β-keto esters has been developed. This method relies on the epimerization of an NHC-enol intermediate before subsequent aldol/acylation events. Highly substituted β-lactones are produced in good yield with good to excellent selectivities (see scheme).
Copyright © 2012 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures
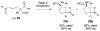

Similar articles
-
N-heterocyclic-carbene-catalyzed asymmetric oxidative hetero-Diels-Alder reactions with simple aliphatic aldehydes.Angew Chem Int Ed Engl. 2012 Dec 3;51(49):12330-3. doi: 10.1002/anie.201206490. Epub 2012 Nov 4. Angew Chem Int Ed Engl. 2012. PMID: 23124903 Free PMC article. No abstract available.
-
N-heterocyclic carbene-catalyzed Ireland-Coates Claisen rearrangement: synthesis of functionalized β-lactones.J Am Chem Soc. 2013 Jan 9;135(1):58-61. doi: 10.1021/ja310449k. Epub 2012 Dec 20. J Am Chem Soc. 2013. PMID: 23244238
-
Intermolecular dynamic kinetic resolution cooperatively catalyzed by an N-heterocyclic carbene and a Lewis acid.Angew Chem Int Ed Engl. 2015 Jan 26;54(5):1629-33. doi: 10.1002/anie.201410030. Epub 2014 Nov 28. Angew Chem Int Ed Engl. 2015. PMID: 25447028
-
[Development of C-C bond formation and asymmetric reactions catalyzed by N-heterocyclic carbenes].Yakugaku Zasshi. 2008 Aug;128(8):1179-85. doi: 10.1248/yakushi.128.1179. Yakugaku Zasshi. 2008. PMID: 18670183 Review. Japanese.
-
The chemistry of functionalised N-heterocyclic carbenes.Chem Soc Rev. 2007 Apr;36(4):592-607. doi: 10.1039/b603765h. Epub 2006 Sep 8. Chem Soc Rev. 2007. PMID: 17387408 Review.
Cited by
-
Catalytic Kinetic Resolution of a Dynamic Racemate: Highly Stereoselective β-Lactone Formation by N-Heterocyclic Carbene Catalysis.Chem Sci. 2014 May 1;5(5):1974-1982. doi: 10.1039/C4SC00317A. Chem Sci. 2014. PMID: 25045464 Free PMC article.
-
Functionalized cyclopentenes through a tandem NHC-catalyzed dynamic kinetic resolution and ambient temperature decarboxylation: mechanistic insight and synthetic application.Chem Commun (Camb). 2015 Feb 14;51(13):2690-3. doi: 10.1039/c4cc09308a. Chem Commun (Camb). 2015. PMID: 25575249 Free PMC article.
-
Stereoconvergent Conjugate Addition of Arylboronic Acids to α-Angelica Lactone Derivatives: Synthesis of Stereochemically Complex γ-Butyrolactones.ACS Catal. 2019 Dec 6;9(12):11614-11618. doi: 10.1021/acscatal.9b04405. Epub 2019 Nov 19. ACS Catal. 2019. PMID: 33815896 Free PMC article.
-
Synthesis of Complex Tertiary Glycolates by Enantioconvergent Arylation of Stereochemically Labile α-Keto Esters.J Am Chem Soc. 2017 Mar 15;139(10):3911-3916. doi: 10.1021/jacs.7b00943. Epub 2017 Mar 2. J Am Chem Soc. 2017. PMID: 28252953 Free PMC article.
-
Direct Zinc(II)-Catalyzed Enantioconvergent Additions of Terminal Alkynes to α-Keto Esters.Angew Chem Int Ed Engl. 2017 Jul 17;56(30):8805-8808. doi: 10.1002/anie.201704226. Epub 2017 Jun 20. Angew Chem Int Ed Engl. 2017. PMID: 28557339 Free PMC article.
References
-
- Kagan HB, Fiaud JC. Topics in Stereochemistry. John Wiley & Sons, Inc; 1988. p. 249.
- Keith JM, Larrow JF, Jacobsen EN. Adv Synth Catal. 2001;343:5.
- Krasnov VP, Gruzdev DA, Levit GL. Eur J Org Chem. 2012;2012:1471.
- Müller CE, Schreiner PR. Angew Chem Int Ed. 2011;50:6012. - PubMed
- Taylor JE, Bull SD, Williams JMJ. Chem Soc Rev. 2012;41:2109. - PubMed
- Vedejs E, Jure M. Angew Chem Int Ed. 2005;44:3974. - PubMed
-
-
For recent advances, see: Peschiulli A, Procuranti B, Connor CJ, O’Connon SJ. Nat Chem. 2010;2:380.Bumbu VD, Birman VB. J Am Chem Soc. 2011;133:13902.Coric I, List B. Nature. 2012;486:315.Wang Y, Zheng K, Hong R. J Am Chem Soc. 2012;134:4096.
-
-
- Noyori R, Tokunaga M, Kitamura M. Bull Chem Soc Jpn. 1995;68:36.
- Huerta FF, Minidis ABE, Bäckvall JE. Chem Soc Rev. 2001;30:321.
- Tian SK, Chen Y, Hang J, Tang L, McDaid P, Deng L. Acc Chem Res. 2004;37:621. - PubMed
- Pellissier H. Adv Synth Catal. 2011;353:659.
-
-
For dynamic kinetic resolutions using a chiral auxiliary, see: Amat M, Cantó M, Llor N, Ponzo V, Pérez M, Bosch J. Angew Chem Int Ed. 2002;41:335.Ito T, Overman LE, Wang J. J Am Chem Soc. 2010;132:3272.Jang JI, Kang SY, Kang KH, Park YS. Tetrahedron. 2011;67:6221.
-
-
-
For selected examples of metal-catalyzed DKRs, see: Noyori R, Ikeda T, Ohkuma T, Widhalm M, Kitamura M, Takaya H, Akutagawa S, Sayo N, Saito T. J Am Chem Soc. 1989;111:9134.Kitamura M, Tokunaga M, Noyori R. J Am Chem Soc. 1993;115:144.Trost BM, Andersen NG. J Am Chem Soc. 2002;124:14320B.Martín-Matute, Edin M, Bogár K, Kaynak FB, Bäckvall J-E. J Am Chem Soc. 2005;127:8817.Rainka MP, Milne JE, Buchwald SL. Angew Chem Int Ed. 2005;44:6177.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

