Use of kinase inhibitors to correct ΔF508-CFTR function
- PMID: 22700489
- PMCID: PMC3434788
- DOI: 10.1074/mcp.M111.016626
Use of kinase inhibitors to correct ΔF508-CFTR function
Abstract
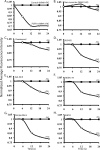
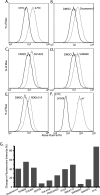
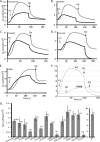
The most common mutation in cystic fibrosis (CF) is a deletion of Phe at position 508 (ΔF508-CFTR). ΔF508-CFTR is a trafficking mutant that is retained in the ER, unable to reach the plasma membrane. To identify compounds and drugs that rescue this trafficking defect, we screened a kinase inhibitor library enriched for small molecules already in the clinic or in clinical trials for the treatment of cancer and inflammation, using our recently developed high-content screen technology (Trzcinska-Daneluti et al. Mol. Cell. Proteomics 8:780, 2009). The top hits of the screen were further validated by (1) biochemical analysis to demonstrate the presence of mature (Band C) ΔF508-CFTR, (2) flow cytometry to reveal the presence of ΔF508-CFTR at the cell surface, (3) short-circuit current (Isc) analysis in Ussing chambers to show restoration of function of the rescued ΔF508-CFTR in epithelial MDCK cells stably expressing this mutant (including EC(50) determinations), and importantly (4) Isc analysis of Human Bronchial Epithelial (HBE) cells harvested from homozygote ΔF508-CFTR transplant patients. Interestingly, several inhibitors of receptor Tyr kinases (RTKs), such as SU5402 and SU6668 (which target FGFRs, VEGFR, and PDGFR) exhibited strong rescue of ΔF508-CFTR, as did several inhibitors of the Ras/Raf/MEK/ERK or p38 pathways (e.g. (5Z)-7-oxozeaenol). Prominent rescue was also observed by inhibitors of GSK-3β (e.g. GSK-3β Inhibitor II and Kenpaullone). These results identify several kinase inhibitors that can rescue ΔF508-CFTR to various degrees, and suggest that use of compounds or drugs already in the clinic or in clinical trials for other diseases can expedite delivery of treatment for CF patients.
Figures



References
-
- Boucher R. C. (2007) Airway surface dehydration in cystic fibrosis: pathogenesis and therapy. Annu. Rev. Med. 58, 157–170 - PubMed
-
- Donaldson S. H., Boucher R. C. (2007) Sodium channels and cystic fibrosis. Chest 132, 1631–1636 - PubMed
-
- Ratjen F. A. (2009) Cystic fibrosis: pathogenesis and future treatment strategies. Respir. Care 54, 595–605 - PubMed
-
- Riordan J. R. (2008) CFTR function and prospects for therapy. Annu. Rev. Biochem. 77, 701–726 - PubMed
-
- Riordan J. R. (2005) Assembly of functional CFTR chloride channels. Annu. Rev. Physiol. 67, 701–718 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

