uPA binding to PAI-1 induces corneal myofibroblast differentiation on vitronectin
- PMID: 22700714
- PMCID: PMC3949353
- DOI: 10.1167/iovs.12-10042
uPA binding to PAI-1 induces corneal myofibroblast differentiation on vitronectin
Abstract
Purpose: Vitronectin (VN) in provisional extracellular matrix (ECM) promotes cell migration. Fibrotic ECM also includes VN and, paradoxically, strongly adherent myofibroblasts (Mfs). Because fibrotic Mfs secrete elevated amounts of urokinase plasminogen activator (uPA), we tested whether increased extracellular uPA promotes the persistence of Mfs on VN.
Methods: Primary human corneal fibroblasts (HCFs) were cultured in supplemented serum-free medium on VN or collagen (CL) with 1 ng/mL transforming growth factor β1 (TGFβ1). Adherent cells were quantified using crystal violet. Protein expression was measured by Western blotting and flow cytometry. Transfection of short interfering RNAs was performed by nucleofection. Mfs were identified by α-smooth muscle actin (α-SMA) stress fibers. Plasminogen activator inhibitor (PAI-1) levels were quantified by ELISA.
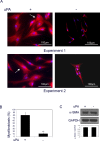
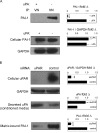
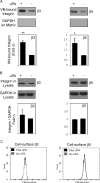
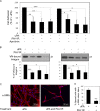
Results: TGFβ1-treated HCFs secreted PAI-1 (0.5 uM) that bound to VN, competing with αvβ3/αvβ5 integrin/VN binding, thus promoting cell detachment from VN. However, addition of uPA to cells on VN increased Mf differentiation (9.7-fold), cell-adhesion (2.2-fold), and binding by the VN integrins αvβ3 and -β5 (2.2-fold). Plasmin activity was not involved in promoting these changes, as treatment with the plasmin inhibitor aprotinin had no effect. A dominant negative PAI-1 mutant (PAI-1R) that binds to VN but does not inhibit uPA prevented the increase in uPA-stimulated cell adhesion and reduced uPA-stimulated integrin αvβ3/αvβ5 binding to VN by 73%.
Conclusions: uPA induction of TGFβ1-dependent Mf differentiation on VN supports the hypothesis that elevated secretion of uPA in fibrotic tissue may promote cell adhesion and the persistence of Mfs. By blocking uPA-stimulated cell adhesion, PAI-1R may be a useful agent in combating corneal scarring.
Conflict of interest statement
Disclosure:
Figures

Similar articles
-
Degradation of internalized αvβ5 integrin is controlled by uPAR bound uPA: effect on β1 integrin activity and α-SMA stress fiber assembly.PLoS One. 2012;7(3):e33915. doi: 10.1371/journal.pone.0033915. Epub 2012 Mar 21. PLoS One. 2012. PMID: 22470492 Free PMC article.
-
Wound-induced migration of MDA-MB-435 and SKOV-3 cancer cells is regulated by plasminogen activator inhibitor-1.Int J Oncol. 2005 Sep;27(3):749-57. Int J Oncol. 2005. PMID: 16077925
-
Plasminogen activator inhibitor-1 detaches cells from extracellular matrices by inactivating integrins.J Cell Biol. 2003 Mar 3;160(5):781-91. doi: 10.1083/jcb.200208117. J Cell Biol. 2003. PMID: 12615913 Free PMC article.
-
Interaction of plasminogen activator inhibitor type-1 (PAI-1) with vitronectin (Vn): mapping the binding sites on PAI-1 and Vn.Biol Chem. 2002 Jul-Aug;383(7-8):1143-9. doi: 10.1515/BC.2002.125. Biol Chem. 2002. PMID: 12437099 Review.
-
Regulation of cell adhesion by PAI-1.APMIS. 1999 Jan;107(1):54-61. doi: 10.1111/j.1699-0463.1999.tb01526.x. APMIS. 1999. PMID: 10190280 Review.
Cited by
-
SLURP-1 modulates corneal homeostasis by serving as a soluble scavenger of urokinase-type plasminogen activator.Invest Ophthalmol Vis Sci. 2014 Aug 28;55(10):6251-61. doi: 10.1167/iovs.14-15107. Invest Ophthalmol Vis Sci. 2014. PMID: 25168896 Free PMC article.
-
Adipose-PAS interactions in the context of its localised bio-engineering potential (Review).Biomed Rep. 2021 Aug;15(2):70. doi: 10.3892/br.2021.1446. Epub 2021 Jul 5. Biomed Rep. 2021. PMID: 34276988 Free PMC article. Review.
-
Wnt/β-catenin pathway and cell adhesion deregulation in CSDE1-related intellectual disability and autism spectrum disorders.Mol Psychiatry. 2021 Jul;26(7):3572-3585. doi: 10.1038/s41380-021-01072-7. Epub 2021 Apr 19. Mol Psychiatry. 2021. PMID: 33867523
-
Vitronectin deficiency attenuates hepatic fibrosis in a non-alcoholic steatohepatitis-induced mouse model.Int J Exp Pathol. 2019 Apr;100(2):72-82. doi: 10.1111/iep.12306. Epub 2019 Mar 18. Int J Exp Pathol. 2019. PMID: 30887659 Free PMC article.
-
The uPA/uPAR System Orchestrates the Inflammatory Response, Vascular Homeostasis, and Immune System in Fibrosis Progression.Int J Mol Sci. 2023 Jan 16;24(2):1796. doi: 10.3390/ijms24021796. Int J Mol Sci. 2023. PMID: 36675310 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
- 5R24CA095823-04/CA/NCI NIH HHS/United States
- HL55374/HL/NHLBI NIH HHS/United States
- 1S10RR09145-01/RR/NCRR NIH HHS/United States
- HL089407/HL/NHLBI NIH HHS/United States
- P30-EY01867/EY/NEI NIH HHS/United States
- R24 CA095823/CA/NCI NIH HHS/United States
- P01 HL089407/HL/NHLBI NIH HHS/United States
- EY017030/EY/NEI NIH HHS/United States
- R01 EY009414/EY/NEI NIH HHS/United States
- 5 U42 RR006042/RR/NCRR NIH HHS/United States
- R01 EY017030/EY/NEI NIH HHS/United States
- R56 EY009414/EY/NEI NIH HHS/United States
- U42 RR006042/RR/NCRR NIH HHS/United States
- R01 HL055374/HL/NHLBI NIH HHS/United States
- P30 EY001867/EY/NEI NIH HHS/United States
- EY09414/EY/NEI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Miscellaneous

