DC-SIGN, C1q, and gC1qR form a trimolecular receptor complex on the surface of monocyte-derived immature dendritic cells
- PMID: 22700724
- PMCID: PMC3418718
- DOI: 10.1182/blood-2011-07-369728
DC-SIGN, C1q, and gC1qR form a trimolecular receptor complex on the surface of monocyte-derived immature dendritic cells
Abstract
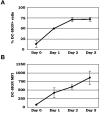
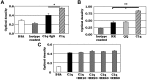
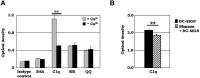
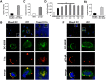
C1q modulates the differentiation and function of cells committed to the monocyte-derived dendritic cell (DC) lineage. Because the 2 C1q receptors found on the DC surface-gC1qR and cC1qR-lack a direct conduit into intracellular elements, we postulated that the receptors must form complexes with transmembrane partners. In the present study, we show that DC-SIGN, a C-type lectin expressed on DCs, binds directly to C1q, as assessed by ELISA, flow cytometry, and immunoprecipitation experiments. Surface plasmon resonance analysis revealed that the interaction was specific, and both intact C1q and the globular portion of C1q bound to DC-SIGN. Whereas IgG reduced this binding significantly, the Arg residues (162-163) of the C1q-A chain, which are thought to contribute to the C1q-IgG interaction, were not required for C1q binding to DC-SIGN. Binding was reduced significantly in the absence of Ca(2+) and by preincubation of DC-SIGN with mannan, suggesting that C1q binds to DC-SIGN at its principal Ca(2+)-binding pocket, which has increased affinity for mannose residues. Antigen-capture ELISA and immunofluorescence microscopy revealed that C1q and gC1qR associate with DC-SIGN on blood DC precursors and immature DCs. The results of the present study suggest that C1q/gC1qR may regulate DC differentiation and function through the DC-SIGN-mediated induction of cell-signaling pathways.
Figures



References
-
- Castellano G, Woltman AM, Nauta AJ, et al. Maturation of dendritic cells abrogates C1q production in vivo and in vitro. Blood. 2004;103:3813–3820. - PubMed
-
- Kaul M, Loos M. Expression of membrane C1q in human monocyte-derived macrophages is developmentally regulated and enhanced by interferon-gamma. FEBS Lett. 2001;500(1-2):91–98. - PubMed
-
- Reid KB. Chemistry and molecular genetics of C1q. Behring Inst Mitt. 1989;84:8–19. - PubMed
-
- Lu J, Wiedemann H, Timpl R, Reid KB. Similarity in structure between C1q and the collectins as judged by electron microscopy. Behring Inst Mitt. 1993;93:6–16. - PubMed
-
- Bobak DA, Gaither TA, Frank MM, Tenner AJ. Modulation of FcR function by complement: subcomponent C1q enhances the phagocytosis of IgG-opsonized targets by human monocytes and culture-derived macrophages. J Immunol. 1987;138(4):1150–1156. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

