CD38 deficiency in the tumor microenvironment attenuates glioma progression and modulates features of tumor-associated microglia/macrophages
- PMID: 22700727
- PMCID: PMC3408254
- DOI: 10.1093/neuonc/nos121
CD38 deficiency in the tumor microenvironment attenuates glioma progression and modulates features of tumor-associated microglia/macrophages
Abstract
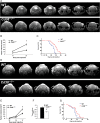
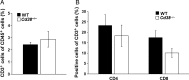
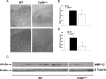
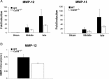
Gliomas are the most frequent primary tumors of the brain, and for highly malignant gliomas there is no successful treatment. The tumor microenvironment contains large numbers of infiltrating microglia and macrophages (MM). There is increasing evidence that the tumor-associated MM support glioma expansion. CD38 is a multifunctional ectoenzyme that uses nicotinamide adenine dinucleotide as a substrate to generate second messengers. Previously we showed that CD38 deficiency modulates microglial "activation" and impaired recovery from head trauma by a microglia-associated mechanism. In view of the supportive role of MM in glioma progression and the role of CD38 in microglia activation, we hypothesize that deficiency of CD38 in the tumor microenvironment would inhibit glioma progression. Using the syngeneic GL261 model of glioma progression in wild-type and CD38 null mice, we show here that CD38 deficiency significantly attenuates glioma expansion and prolongs the life span of the glioma-bearing mice. The CD38 deficiency effect was associated with increased cell death and decreased metalloproteinase-12 expression in the tumor mass, as well as modulation of the tumor-induced MM properties, as indicated by a reduction in the expression of the MM marker F4/80 and matrix metalloproteinases. Our results thus suggest that CD38 participates in the tumor-supporting action of MM and that targeting CD38 might be a potential therapeutic approach for glioma treatment.
Figures



Similar articles
-
Inhibition of glioma progression by a newly discovered CD38 inhibitor.Int J Cancer. 2015 Mar 15;136(6):1422-33. doi: 10.1002/ijc.29095. Epub 2014 Aug 7. Int J Cancer. 2015. PMID: 25053177
-
When Immune Cells Turn Bad-Tumor-Associated Microglia/Macrophages in Glioma.Int J Mol Sci. 2018 Feb 1;19(2):436. doi: 10.3390/ijms19020436. Int J Mol Sci. 2018. PMID: 29389898 Free PMC article. Review.
-
Deletion of the RNA regulator HuR in tumor-associated microglia and macrophages stimulates anti-tumor immunity and attenuates glioma growth.Glia. 2019 Dec;67(12):2424-2439. doi: 10.1002/glia.23696. Epub 2019 Aug 10. Glia. 2019. PMID: 31400163 Free PMC article.
-
Molecular interactions between tumor and its microenvironment in malignant gliomas.Postepy Biochem. 2018 Oct 15;64(2):129-140. doi: 10.18388/pb.2018_123. Postepy Biochem. 2018. PMID: 30656895 Review. English.
-
Glioma-associated microglial MMP9 expression is upregulated by TLR2 signaling and sensitive to minocycline.Int J Cancer. 2014 Dec 1;135(11):2569-78. doi: 10.1002/ijc.28908. Epub 2014 May 9. Int J Cancer. 2014. PMID: 24752463 Free PMC article.
Cited by
-
Drug Delivery Systems in the Development of Novel Strategies for Glioblastoma Treatment.Pharmaceutics. 2022 Jun 1;14(6):1189. doi: 10.3390/pharmaceutics14061189. Pharmaceutics. 2022. PMID: 35745762 Free PMC article. Review.
-
The Good, the Bad and the Unknown of CD38 in the Metabolic Microenvironment and Immune Cell Functionality of Solid Tumors.Cells. 2019 Dec 24;9(1):52. doi: 10.3390/cells9010052. Cells. 2019. PMID: 31878283 Free PMC article. Review.
-
IFN-γ and CD38 in Hyperprogressive Cancer Development.Cancers (Basel). 2021 Jan 15;13(2):309. doi: 10.3390/cancers13020309. Cancers (Basel). 2021. PMID: 33467713 Free PMC article. Review.
-
Decoding NAD+ Metabolism in COVID-19: Implications for Immune Modulation and Therapy.Vaccines (Basel). 2024 Dec 24;13(1):1. doi: 10.3390/vaccines13010001. Vaccines (Basel). 2024. PMID: 39852780 Free PMC article. Review.
-
Exploring the causal relationship between immune factors and chondrosarcoma: a Mendelian randomization study.Discov Oncol. 2025 May 18;16(1):801. doi: 10.1007/s12672-025-02654-5. Discov Oncol. 2025. PMID: 40382743 Free PMC article.
References
-
- Badie B, Schartner J. Role of microglia in glioma biology. Microsc Res Tech. 2001;54:106–113. doi:10.1002/jemt.1125. - DOI - PubMed
-
- Rivest S. Regulation of innate immune responses in the brain. Nat Rev Immunol. 2009;9:429–439. doi:10.1038/nri2565. - DOI - PubMed
-
- Watters JJ, Schartner JM, Badie B. Microglia function in brain tumors. J Neurosci Res. 2005;81:447–455. doi:10.1002/jnr.20485. - DOI - PubMed
-
- Yang I, Han SJ, Kaur G, Crane C, Parsa AT. The role of microglia in central nervous system immunity and glioma immunology. J Clin Neurosci. 2010;17:6–10. doi:10.1016/j.jocn.2009.05.006. - DOI - PMC - PubMed
-
- Markovic DS, Glass R, Synowitz M, Rooijen N, Kettenmann H. Microglia stimulate the invasiveness of glioma cells by increasing the activity of metalloprotease-2. J Neuropathol Exp Neurol. 2005;64:754–762. doi:10.1097/01.jnen.0000178445.33972.a9. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

