WNT1-inducible signaling pathway protein 1 contributes to ventilator-induced lung injury
- PMID: 22700866
- PMCID: PMC3488625
- DOI: 10.1165/rcmb.2012-0127OC
WNT1-inducible signaling pathway protein 1 contributes to ventilator-induced lung injury
Abstract
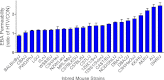
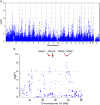
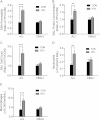
Although strides have been made to reduce ventilator-induced lung injury (VILI), critically ill patients can vary in sensitivity to VILI, suggesting gene-environment interactions could contribute to individual susceptibility. This study sought to uncover candidate genes associated with VILI using a genome-wide approach followed by functional analysis of the leading candidate in mice. Alveolar-capillary permeability after high tidal volume (HTV) ventilation was measured in 23 mouse strains, and haplotype association mapping was performed. A locus was identified on chromosome 15 that contained ArfGAP with SH3 domain, ankyrin repeat and PH domain 1 (Asap1), adenylate cyclase 8 (Adcy8), WNT1-inducible signaling pathway protein 1 (Wisp1), and N-myc downstream regulated 1 (Ndrg1). Information from published studies guided initial assessment to Wisp1. After HTV, lung WISP1 protein increased in sensitive A/J mice, but was unchanged in resistant CBA/J mice. Anti-WISP1 antibody decreased HTV-induced alveolar-capillary permeability in sensitive A/J mice, and recombinant WISP1 protein increased HTV-induced alveolar-capillary permeability in resistant CBA/J mice. HTV-induced WISP1 coimmunoprecipitated with glycosylated Toll-like receptor (TLR) 4 in A/J lung homogenates. After HTV, WISP1 increased in strain-matched control lungs, but was unchanged in TLR4 gene-targeted lungs. In peritoneal macrophages from strain-matched mice, WISP1 augmented LPS-induced TNF release that was inhibited in macrophages from TLR4 or CD14 antigen gene-targeted mice, and was attenuated in macrophages from myeloid differentiation primary response gene 88 gene-targeted or TLR adaptor molecule 1 mutant mice. These findings support a role for WISP1 as an endogenous signal that acts through TLR4 signaling to increase alveolar-capillary permeability in VILI.
Figures




Similar articles
-
WISP1 and TLR4 on Macrophages Contribute to Ventilator-Induced Lung Injury.Inflammation. 2020 Apr;43(2):425-432. doi: 10.1007/s10753-019-01103-0. Inflammation. 2020. PMID: 32130574 Free PMC article.
-
Mechanical ventilation enhances extrapulmonary sepsis-induced lung injury: role of WISP1-αvβ5 integrin pathway in TLR4-mediated inflammation and injury.Crit Care. 2018 Nov 16;22(1):302. doi: 10.1186/s13054-018-2237-0. Crit Care. 2018. PMID: 30445996 Free PMC article.
-
Non‑canonical Wnt signaling contributes to ventilator‑induced lung injury through upregulation of WISP1 expression.Int J Mol Med. 2019 Mar;43(3):1217-1228. doi: 10.3892/ijmm.2019.4067. Epub 2019 Jan 17. Int J Mol Med. 2019. PMID: 30664165 Free PMC article.
-
CCN4/WISP1 (WNT1 inducible signaling pathway protein 1): a focus on its role in cancer.Int J Biochem Cell Biol. 2015 May;62:142-6. doi: 10.1016/j.biocel.2015.03.007. Epub 2015 Mar 17. Int J Biochem Cell Biol. 2015. PMID: 25794425 Review.
-
WISP1: Clinical insights for a proliferative and restorative member of the CCN family.Curr Neurovasc Res. 2014;11(4):378-89. doi: 10.2174/1567202611666140912115107. Curr Neurovasc Res. 2014. PMID: 25219658 Free PMC article. Review.
Cited by
-
TLR4 signaling induces TLR3 up-regulation in alveolar macrophages during acute lung injury.Sci Rep. 2017 Feb 15;7:34278. doi: 10.1038/srep34278. Sci Rep. 2017. PMID: 28198368 Free PMC article.
-
WISP1 mediates hepatic warm ischemia reperfusion injury via TLR4 signaling in mice.Sci Rep. 2016 Jan 29;6:20141. doi: 10.1038/srep20141. Sci Rep. 2016. PMID: 26821752 Free PMC article.
-
Optimal CCN4 Immunofluorescence for Tissue Microarray.Methods Mol Biol. 2023;2582:13-21. doi: 10.1007/978-1-0716-2744-0_2. Methods Mol Biol. 2023. PMID: 36370340
-
Neutrophil Extracellular Traps Are Pathogenic in Ventilator-Induced Lung Injury and Partially Dependent on TLR4.Biomed Res Int. 2017;2017:8272504. doi: 10.1155/2017/8272504. Epub 2017 Dec 13. Biomed Res Int. 2017. PMID: 29387725 Free PMC article.
-
WISP1 polymorphisms contribute to platinum-based chemotherapy toxicity in lung cancer patients.Int J Mol Sci. 2014 Nov 14;15(11):21011-27. doi: 10.3390/ijms151121011. Int J Mol Sci. 2014. PMID: 25405734 Free PMC article.
References
-
- Rubenfeld GD, Caldwell E, Peabody E, Weaver J, Martin DP, Neff M, Stern EJ, Hudson LD. Incidence and outcomes of acute lung injury. N Engl J Med 2005;353:1685–1693 - PubMed
-
- Wheeler AP, Bernard GR. Acute lung injury and the acute respiratory distress syndrome: a clinical review. Lancet 2007;369:1553–1564 - PubMed
-
- Dolinay T, Kaminski N, Felgendreher M, Kim HP, Reynolds P, Watkins SC, Karp D, Uhlig S, Choi AM. Gene expression profiling of target genes in ventilator-induced lung injury. Physiol Genomics 2006;26:68–75 - PubMed
-
- Ma SF, Grigoryev DN, Taylor AD, Nonas S, Sammani S, Ye SQ, Garcia JG. Bioinformatic identification of novel early stress response genes in rodent models of lung injury. Am J Physiol Lung Cell Mol Physiol 2005;289:L468–L477 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 HL077763/HL/NHLBI NIH HHS/United States
- GM080906/GM/NIGMS NIH HHS/United States
- R01 DK083752/DK/NIDDK NIH HHS/United States
- R01 GM080906/GM/NIGMS NIH HHS/United States
- HL65697/HL/NHLBI NIH HHS/United States
- R01 GM078238/GM/NIGMS NIH HHS/United States
- GM50441/GM/NIGMS NIH HHS/United States
- R37 HL065697/HL/NHLBI NIH HHS/United States
- R01 HL085655/HL/NHLBI NIH HHS/United States
- K08 HL079456/HL/NHLBI NIH HHS/United States
- HL085655/HL/NHLBI NIH HHS/United States
- HL79456/HL/NHLBI NIH HHS/United States
- HL077763/HL/NHLBI NIH HHS/United States
- R01 GM050441/GM/NIGMS NIH HHS/United States
- U01 ES015675/ES/NIEHS NIH HHS/United States
- ES015675/ES/NIEHS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

