Protein secretion and the endoplasmic reticulum
- PMID: 22700933
- PMCID: PMC3405867
- DOI: 10.1101/cshperspect.a012872
Protein secretion and the endoplasmic reticulum
Abstract
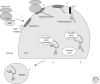
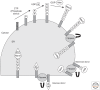
In a complex multicellular organism, different cell types engage in specialist functions, and as a result, the secretory output of cells and tissues varies widely. Whereas some quiescent cell types secrete minor amounts of proteins, tissues like the pancreas, producing insulin and other hormones, and mature B cells, producing antibodies, place a great demand on their endoplasmic reticulum (ER). Our understanding of how protein secretion in general is controlled in the ER is now quite sophisticated. However, there remain gaps in our knowledge, particularly when applying insight gained from model systems to the more complex situations found in vivo. This article describes recent advances in our understanding of the ER and its role in preparing proteins for secretion, with an emphasis on glycoprotein quality control and pathways of disulfide bond formation.
Figures


References
-
- Anelli T, Bergamelli L, Margittai E, Rimessi A, Fagioli C, Malgaroli A, Pinton P, Ripamonti M, Rizzuto R, Sitia R 2012. Ero1α regulates Ca2+ fluxes at the endoplasmic reticulum-mitochondria interface (MAM). Antioxid Redox Signal 16: 1077–1087 - PubMed
-
- Appenzeller-Herzog C 2011. Glutathione- and non-glutathione-based oxidant control in the endoplasmic reticulum. J Cell Sci 124: 847–855 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
