Chemical synthesis of circular proteins
- PMID: 22700959
- PMCID: PMC3411039
- DOI: 10.1074/jbc.R111.323568
Chemical synthesis of circular proteins
Abstract
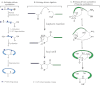
Circular proteins, once thought to be rare, are now commonly found in plants. Their chemical synthesis, once thought to be difficult, is now readily achievable. The enabling methodology is largely due to the advances in entropic chemical ligation to overcome the entropy barrier in coupling the N- and C-terminal ends of large peptide segments for either intermolecular ligation or intramolecular ligation in end-to-end cyclization. Key elements of an entropic chemical ligation consist of a chemoselective capture step merging the N and C termini as a covalently linked O/S-ester intermediate to permit the subsequent step of an intramolecular O/S-N acyl shift to form an amide. Many ligation methods exploit the supernucleophilicity of a thiol side chain at the N terminus for the capture reaction, which makes cysteine-rich peptides ideal candidates for the entropy-driven macrocyclization. Advances in desulfurization and modification of the thiol-containing amino acids at the ligation sites to other amino acids add extra dimensions to the entropy-driven ligation methods. This minireview describes recent advances of entropy-driven ligation to prepare circular proteins with or without a cysteinyl side chain.
Figures
References
-
- Henriques S. T., Craik D. J. (2010) Cyclotides as templates in drug design. Drug Discov. Today 15, 57–64 - PubMed
-
- Saska I., Gillon A. D., Hatsugai N., Dietzgen R. G., Hara-Nishimura I., Anderson M. A., Craik D. J. (2007) An asparaginyl endopeptidase mediates in vivo protein backbone cyclization. J. Biol. Chem. 282, 29721–29728 - PubMed
-
- Gran L. (1973) On the effect of a polypeptide isolated from “Kalata-Kalata” (Oldenlandia affinis DC) on the estrogen-dominated uterus. Acta Pharmacol. Toxicol. 33, 400–408 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

