Palmitoleate is a mitogen, formed upon stimulation with growth factors, and converted to palmitoleoyl-phosphatidylinositol
- PMID: 22700983
- PMCID: PMC3411066
- DOI: 10.1074/jbc.M111.274829
Palmitoleate is a mitogen, formed upon stimulation with growth factors, and converted to palmitoleoyl-phosphatidylinositol
Abstract
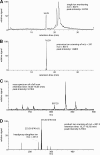
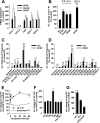
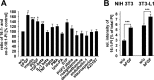
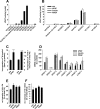
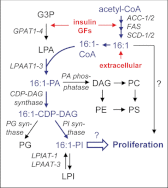
Controversial correlations between biological activity and concentration of the novel lipokine palmitoleate (9Z-hexadecenoate, 16:1) might depend on the formation of an active 16:1 metabolite. For its identification, we analyzed the glycerophospholipid composition of mouse Swiss 3T3 fibroblasts in response to 16:1 using LC-MS/MS. 16:1 was either supplemented to the cell culture medium or endogenously formed when cells were stimulated with insulin or growth factors as suggested by the enhanced mRNA expression of 16:1-biosynthetic enzymes. The proportion of 1-acyl-2-16:1-sn-phosphatidylinositol (16:1-PI) was time-dependently and specifically increased relative to other glycerophospholipids under both conditions and correlated with the proliferation of fatty acid (16:1, palmitate, oleate, or arachidonate)-supplemented cells. Accordingly, cell proliferation was impaired by blocking 16:1 biosynthesis using the selective stearoyl-CoA desaturase-1 inhibitor CAY10566 and restored by supplementation of 16:1. The accumulation of 16:1-PI occurred throughout cellular compartments and within diverse mouse cell lines (Swiss 3T3, NIH-3T3, and 3T3-L1 cells). To elucidate further whether 16:1-PI is formed through the de novo or remodeling pathway of PI biosynthesis, phosphatidate levels and lyso-PI-acyltransferase activities were analyzed as respective markers. The proportion of 16:1-phosphatidate was significantly increased by insulin and growth factors, whereas lyso-PI-acyltransferases showed negligible activity for 16:1-coenzyme A. The relevance of the de novo pathway for 16:1-PI biosynthesis is supported further by the comparable incorporation rate of deuterium-labeled 16:1 and tritium-labeled inositol into PI for growth factor-stimulated cells. In conclusion, we identified 16:1 or 16:1-PI as mitogen whose biosynthesis is induced by growth factors.
Figures

References
-
- Shindou H., Shimizu T. (2009) Acyl-CoA:lysophospholipid acyltransferases. J. Biol. Chem. 284, 1–5 - PubMed
-
- Kent C. (1995) Eukaryotic phospholipid biosynthesis. Annu. Rev. Biochem. 64, 315–343 - PubMed
-
- Warensjö E., Ohrvall M., Vessby B. (2006) Fatty acid composition and estimated desaturase activities are associated with obesity and lifestyle variables in men and women. Nutr. Metab. Cardiovasc. Dis. 16, 128–136 - PubMed
-
- Paillard F., Catheline D., Duff F. L., Bouriel M., Deugnier Y., Pouchard M., Daubert J. C., Legrand P. (2008) Plasma palmitoleic acid, a product of stearoyl-CoA desaturase activity, is an independent marker of triglyceridemia and abdominal adiposity. Nutr. Metab. Cardiovasc. Dis. 18, 436–440 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

