Cell polarization and cytokinesis in budding yeast
- PMID: 22701052
- PMCID: PMC3374305
- DOI: 10.1534/genetics.111.132886
Cell polarization and cytokinesis in budding yeast
Abstract
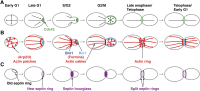
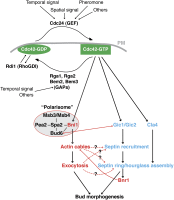
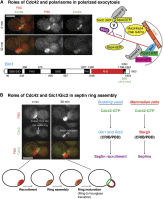
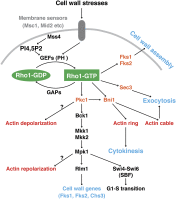
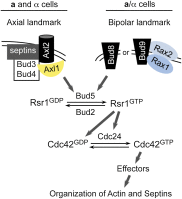
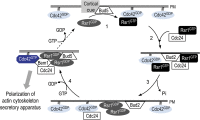
Asymmetric cell division, which includes cell polarization and cytokinesis, is essential for generating cell diversity during development. The budding yeast Saccharomyces cerevisiae reproduces by asymmetric cell division, and has thus served as an attractive model for unraveling the general principles of eukaryotic cell polarization and cytokinesis. Polarity development requires G-protein signaling, cytoskeletal polarization, and exocytosis, whereas cytokinesis requires concerted actions of a contractile actomyosin ring and targeted membrane deposition. In this chapter, we discuss the mechanics and spatial control of polarity development and cytokinesis, emphasizing the key concepts, mechanisms, and emerging questions in the field.
Figures

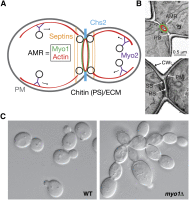
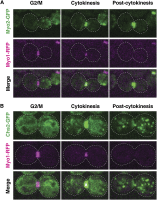
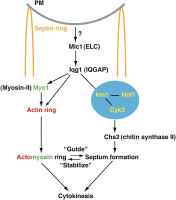
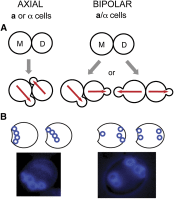
References
-
- Adames N., Blundell K., Ashby M. N., Boone C., 1995. Role of yeast insulin-degrading enzyme homologs in propheromone processing and bud site selection. Science 270: 464–467 - PubMed
-
- Adams A. E., Botstein D., Drubin D. G., 1989. A yeast actin-binding protein is encoded by SAC6, a gene found by suppression of an actin mutation. Science 243: 231–233 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

