An Insight into the Diverse Roles of Surfactant Proteins, SP-A and SP-D in Innate and Adaptive Immunity
- PMID: 22701116
- PMCID: PMC3369187
- DOI: 10.3389/fimmu.2012.00131
An Insight into the Diverse Roles of Surfactant Proteins, SP-A and SP-D in Innate and Adaptive Immunity
Abstract
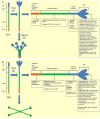
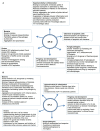
Surfactant proteins SP-A and SP-D are hydrophilic, collagen-containing calcium-dependent lectins, which appear to have a range of innate immune functions at pulmonary as well as extrapulmonary sites. These proteins bind to target ligands on pathogens, allergens, and apoptotic cells, via C-terminal homotrimeric carbohydrate recognition domains, while the collagen region brings about the effector functions via its interaction with cell surface receptors. SP-A and SP-D deal with various pathogens, using a range of innate immune mechanisms such as agglutination/aggregation, enhancement of phagocytosis, and killing mechanisms by phagocytic cells and direct growth inhibition. SP-A and SP-D have also been shown to be involved in the control of pulmonary inflammation including allergy and asthma. Emerging evidence suggest that SP-A and SP-D are capable of linking innate immunity with adaptive immunity that includes modulation of dendritic cell function and helper T cell polarization. This review enumerates immunological properties of SP-A and SP-D inside and outside lungs and discusses their importance in human health and disease.
Keywords: hypersensitivity; infection; innate immunity; macrophage; pattern recognition receptor; surfactant.
Figures
References
LinkOut - more resources
Full Text Sources

