Selective accumulation of Th2-skewing immature erythroid cells in developing neonatal mouse spleen
- PMID: 22701342
- PMCID: PMC3371569
- DOI: 10.7150/ijbs.3764
Selective accumulation of Th2-skewing immature erythroid cells in developing neonatal mouse spleen
Abstract
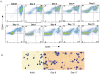
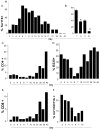
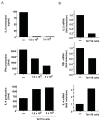
Environmental factors likely regulate neonatal immunity and self-tolerance. However, evidence that the neonatal immune system is suppressed or deviated is varied depending on the antigen and the timing of antigen exposure relative to birth. These disparate findings may be related to the availability of the appropriate antigen presenting cells but also point to the possibility of homeostatic changes in non-lymphoid cells in the relevant lymphoid tissues. Here we show that, while leukocytes are the most abundant cell population present in spleen during the first 4-5 days after birth, a massive accumulation of nucleated immature erythroid population in the spleen takes places on day 6 after birth. Although the relative frequency of these immature erythorid cells slowly decreases during the development of neonates, they remain one of the most predominant populations up to three weeks of age. Importantly, we show that the immature erythroid cells from neonate spleen have the capacity to modulate the differentiation of CD4 T cells into effector cells and provide a bias towards a Th2 type instead of Th1 type. These nucleated erythroid cells can produce cytokines that participate in the Th2/Th1 balance, an important one being IL-6. Thus, the selective accumulation of immature erythroid cells in the spleen during a specific period of neonatal development may explain the apparent differences observed in the type(s) of immune responses generated in infants and neonates. These findings are potentially relevant to the better management of immune deficiency in and to the design of vaccination strategies for the young.
Keywords: Neonatal immunity; T lymphocytes.; erythrocytes.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures


Similar articles
-
Exclusive Th2 primary effector function in spleens but mixed Th1/Th2 function in lymph nodes of murine neonates.J Immunol. 2000 Mar 1;164(5):2347-53. doi: 10.4049/jimmunol.164.5.2347. J Immunol. 2000. PMID: 10679069
-
Newborn mice develop balanced Th1/Th2 primary effector responses in vivo but are biased to Th2 secondary responses.J Immunol. 1998 May 1;160(9):4217-24. J Immunol. 1998. PMID: 9574522
-
Ontogenic development of Th1 and Th2 cytokine capabilities in random bred mice.Dev Immunol. 2002 Mar;9(1):1-8. doi: 10.1080/10446670290030963. Dev Immunol. 2002. PMID: 12353658 Free PMC article.
-
Maturation of human neonatal CD4+ and CD8+ T lymphocytes into Th1/Th2 effectors.Vaccine. 1998 Aug-Sep;16(14-15):1415-9. doi: 10.1016/s0264-410x(98)00101-7. Vaccine. 1998. PMID: 9711781 Review.
-
Development of neonatal Th1/Th2 function.Int Rev Immunol. 2000;19(2-3):157-71. doi: 10.3109/08830180009088503. Int Rev Immunol. 2000. PMID: 10763707 Review.
Cited by
-
CD71+ erythroid cells from neonates born to women with preterm labor regulate cytokine and cellular responses.J Leukoc Biol. 2018 Apr;103(4):761-775. doi: 10.1002/JLB.5A0717-291RRR. Epub 2018 Feb 1. J Leukoc Biol. 2018. PMID: 29389020 Free PMC article.
-
Erythroid Lineage Cells in the Liver: Novel Immune Regulators and Beyond.J Clin Transl Hepatol. 2020 Jun 28;8(2):177-183. doi: 10.14218/JCTH.2019.00031. Epub 2020 Jun 8. J Clin Transl Hepatol. 2020. PMID: 32832398 Free PMC article. Review.
-
Cytokine-Producing B Cells Promote Immune-Mediated Bile Duct Injury in Murine Biliary Atresia.Hepatology. 2018 Nov;68(5):1890-1904. doi: 10.1002/hep.30051. Epub 2018 Jul 10. Hepatology. 2018. PMID: 29679373 Free PMC article.
-
Prenatal Maternal Stress Causes Preterm Birth and Affects Neonatal Adaptive Immunity in Mice.Front Immunol. 2020 Feb 26;11:254. doi: 10.3389/fimmu.2020.00254. eCollection 2020. Front Immunol. 2020. PMID: 32174914 Free PMC article.
-
Erythroblastic island: the niche for erythroid terminal differentiation and beyond.Blood Sci. 2025 Mar 21;7(2):e00228. doi: 10.1097/BS9.0000000000000228. eCollection 2025 Jun. Blood Sci. 2025. PMID: 40129604 Free PMC article. Review.
References
-
- Inter-agency Group for Child Mortaliaty Estimation. Levels and Trends in Child Mortality: Report 2010. UN. 2010.
-
- Yorita KL, Holman RC, Sejvar JJ, Steiner CA, Schonberger LB. Infectious disease hospitalizations among infants in the United States. Pediatrics. 2008;121(2):244–52. - PubMed
-
- Lederberg J. Genes and antibodies. Science. 1959;129(3364):1649–53. - PubMed
-
- Adkins B, Chun K, Hamilton K, Nassiri M. Naive murine neonatal T cells undergo apoptosis in response to primary stimulation. J Immunol. 1996;157(4):1343–1349. - PubMed
-
- Ridge JP, Fuchs EJ, Matzinger P. Neonatal tolerance revisited: turning on newborn T cells with dendritic cells. Science. 1996;271(5256):1723–6. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

