Neutrophil arrest by LFA-1 activation
- PMID: 22701459
- PMCID: PMC3373145
- DOI: 10.3389/fimmu.2012.00157
Neutrophil arrest by LFA-1 activation
Abstract
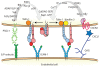
Lymphocyte function-associated antigen-1 (LFA-1) is a heterodimeric integrin consisting of α(L) (gene name, Itgal) and β(2) (gene name, Itgb2) subunits expressed in all leukocytes. LFA-1 is essential for neutrophil recruitment to inflamed tissue. Activation of LFA-1 by chemokines allows neutrophils and other leukocytes to undergo arrest, resulting in firm adhesion on endothelia expressing intercellular adhesion molecules (ICAMs). In mice, CXCR2 is the primary chemokine receptor involved in triggering neutrophil arrest, and it does so through "inside-out" activation of LFA-1. CXCR2 signaling induces changes in LFA-1 conformation that are coupled to affinity upregulation of the ligand-binding headpiece (extended with open I domain). Unlike naïve lymphocytes, engagement of P-selectin glycoprotein ligand-1 (PSGL-1) on neutrophils stimulates a slow rolling behavior that is mediated by LFA-1 in a distinct activation state (extended with closed I domain). How inside-out signaling cascades regulate the structure and function of LFA-1 is being studied using flow chambers, intravital microscopy, and flow cytometry for ligand and reporter antibody binding. Here, we review how LFA-1 activation is regulated by cellular signaling and ligand binding. Two FERM domain-containing proteins, talin-1 and Kindlin-3, are critical integrin co-activators and have distinct roles in the induction of LFA-1 conformational rearrangements. This review integrates these new results into existing models of LFA-1 activation.
Keywords: LFA-1; chemokine; inflammation; integrin; neutrophil.
Figures

References
-
- Alon R., Aker M., Feigelson S., Sokolovsky-Eisenberg M., Staunton D. E., Cinamon G., Grabovsky V., Shamri R., Etzioni A. (2003). A novel genetic leukocyte adhesion deficiency in subsecond triggering of integrin avidity by endothelial chemokines results in impaired leukocyte arrest on vascular endothelium under shear flow. Blood 101 4437–4445 - PubMed
-
- Alon R., Dustin M. L. (2007). Force as a facilitator of integrin conformational changes during leukocyte arrest on blood vessels and antigen-presenting cells. Immunity 26 17–27 - PubMed
-
- Alon R., Etzioni A. (2003). LAD-III, a novel group of leukocyte integrin activation deficiencies. Trends Immunol. 24 561–566 - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

