An Optically Controlled 3D Cell Culturing System
- PMID: 22701475
- PMCID: PMC3373019
- DOI: 10.1155/2011/253989
An Optically Controlled 3D Cell Culturing System
Abstract
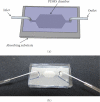
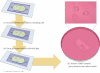
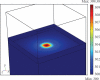
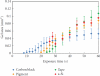
A novel 3D cell culture system was developed and tested. The cell culture device consists of a microfluidic chamber on an optically absorbing substrate. Cells are suspended in a thermoresponsive hydrogel solution, and optical patterns are utilized to heat the solution, producing localized hydrogel formation around cells of interest. The hydrogel traps only the desired cells in place while also serving as a biocompatible scaffold for supporting the cultivation of cells in 3D. This is demonstrated with the trapping of MDCK II and HeLa cells. The light intensity from the optically induced hydrogel formation does not significantly affect cell viability.
Figures



Similar articles
-
Microfluidic Platform for the Long-Term On-Chip Cultivation of Mammalian Cells for Lab-On-A-Chip Applications.Sensors (Basel). 2017 Jul 10;17(7):1603. doi: 10.3390/s17071603. Sensors (Basel). 2017. PMID: 28698531 Free PMC article.
-
Controllable 3D alginate hydrogel patterning via visible-light induced electrodeposition.Biofabrication. 2016 Apr 25;8(2):025004. doi: 10.1088/1758-5090/8/2/025004. Biofabrication. 2016. PMID: 27108617
-
Label-Free On-Chip Selective Extraction of Cell-Aggregate-Laden Microcapsules from Oil into Aqueous Solution with Optical Sensor and Dielectrophoresis.ACS Sens. 2018 Feb 23;3(2):410-417. doi: 10.1021/acssensors.7b00834. Epub 2018 Jan 24. ACS Sens. 2018. PMID: 29299919 Free PMC article.
-
[Use of Porous Hydrogel as a 3D Scaffold for the Growth of Leukemic B Lymphocytes].Klin Onkol. 2017 Spring;30(Supplementum1):184-186. Klin Onkol. 2017. PMID: 28471202 Czech.
-
Fabrication of High-Aspect-Ratio 3D Hydrogel Microstructures Using Optically Induced Electrokinetics.Micromachines (Basel). 2016 Apr 12;7(4):65. doi: 10.3390/mi7040065. Micromachines (Basel). 2016. PMID: 30407438 Free PMC article.
References
-
- Dove A. Cell-based therapies go live. Nature Biotechnology. 2002;20(4):339–343. - PubMed
-
- Wilan KH, Scott CT, Herrera S. Chasing a cellular fountain of youth. Nature Biotechnology. 2005;23(7):807–815. - PubMed
-
- Segers VFM, Lee RT. Stem-cell therapy for cardiac disease. Nature. 2008;451(7181):937–942. - PubMed
-
- Xu W, Liu L, Charles IG. Microencapsulated iNOS-expressing cells cause tumor suppression in mice. The FASEB Journal. 2002;16(2):213–215. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
