Expression of transient receptor potential ankyrin 1 (TRPA1) and its role in insulin release from rat pancreatic beta cells
- PMID: 22701540
- PMCID: PMC3365106
- DOI: 10.1371/journal.pone.0038005
Expression of transient receptor potential ankyrin 1 (TRPA1) and its role in insulin release from rat pancreatic beta cells
Abstract
Objective: Several transient receptor potential (TRP) channels are expressed in pancreatic beta cells and have been proposed to be involved in insulin secretion. However, the endogenous ligands for these channels are far from clear. Here, we demonstrate the expression of the transient receptor potential ankyrin 1 (TRPA1) ion channel in the pancreatic beta cells and its role in insulin release. TRPA1 is an attractive candidate for inducing insulin release because it is calcium permeable and is activated by molecules that are produced during oxidative glycolysis.
Methods: Immunohistochemistry, RT-PCR, and Western blot techniques were used to determine the expression of TRPA1 channel. Ca²⁺ fluorescence imaging and electrophysiology (voltage- and current-clamp) techniques were used to study the channel properties. TRPA1-mediated insulin release was determined using ELISA.
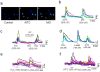
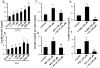
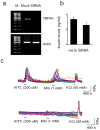
Results: TRPA1 is abundantly expressed in a rat pancreatic beta cell line and freshly isolated rat pancreatic beta cells, but not in pancreatic alpha cells. Activation of TRPA1 by allyl isothiocyanate (AITC), hydrogen peroxide (H₂O₂), 4-hydroxynonenal (4-HNE), and cyclopentenone prostaglandins (PGJ₂) and a novel agonist methylglyoxal (MG) induces membrane current, depolarization, and Ca²⁺ influx leading to generation of action potentials in a pancreatic beta cell line and primary cultured pancreatic beta cells. Activation of TRPA1 by agonists stimulates insulin release in pancreatic beta cells that can be inhibited by TRPA1 antagonists such as HC030031 or AP-18 and by RNA interference. TRPA1-mediated insulin release is also observed in conditions of voltage-gated Na⁺ and Ca²⁺ channel blockade as well as ATP sensitive potassium (K(ATP)) channel activation.
Conclusions: We propose that endogenous and exogenous ligands of TRPA1 cause Ca²⁺ influx and induce basal insulin release and that TRPA1-mediated depolarization acts synergistically with K(ATP) channel blockade to facilitate insulin release.
Conflict of interest statement
Figures

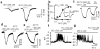
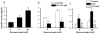
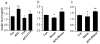
References
-
- Nilius B, Owsianik G, Voets T, Peters JA. Transient receptor potential cation channels in disease. Physiol Rev. 2007;87:165–217. - PubMed
-
- Razavi R, Chan Y, Afifiyan, Liu XJ, Wan X, et al. TRPV1+ sensory neurons control beta cell stress and islet inflammation in autoimmune diabetes. Cell. 2006;127:1123–1135. - PubMed
-
- Gram DX, Ahrén B, Nagy, Olsen UB, Brand CL, et al. Capsaicin-sensitive sensory fibers in the islets of Langerhans contribute to defective insulin secretion in Zucker diabetic rat, an animal model for some aspects of human type 2 diabetes. Eur J Neurosci. 2007;25:213–223. - PubMed
-
- Akiba Y, Kato S, Katsube K, Nakamura M, Takeuchi K, et al. Transient receptor potential vanilloid subfamily 1 expressed in pancreatic islet beta cells modulates insulin secretion in rats. Biochem Biophys Res Commun. 2004;321:219–225. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

