RNA folding and catalysis mediated by iron (II)
- PMID: 22701543
- PMCID: PMC3365117
- DOI: 10.1371/journal.pone.0038024
RNA folding and catalysis mediated by iron (II)
Abstract
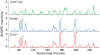
Mg²⁺ shares a distinctive relationship with RNA, playing important and specific roles in the folding and function of essentially all large RNAs. Here we use theory and experiment to evaluate Fe²⁺ in the absence of free oxygen as a replacement for Mg²⁺ in RNA folding and catalysis. We describe both quantum mechanical calculations and experiments that suggest that the roles of Mg²⁺ in RNA folding and function can indeed be served by Fe²⁺. The results of quantum mechanical calculations show that the geometry of coordination of Fe²⁺ by RNA phosphates is similar to that of Mg²⁺. Chemical footprinting experiments suggest that the conformation of the Tetrahymena thermophila Group I intron P4-P6 domain RNA is conserved between complexes with Fe²⁺ or Mg²⁺. The catalytic activities of both the L1 ribozyme ligase, obtained previously by in vitro selection in the presence of Mg²⁺, and the hammerhead ribozyme are enhanced in the presence of Fe²⁺ compared to Mg²⁺. All chemical footprinting and ribozyme assays in the presence of Fe²⁺ were performed under anaerobic conditions. The primary motivation of this work is to understand RNA in plausible early earth conditions. Life originated during the early Archean Eon, characterized by a non-oxidative atmosphere and abundant soluble Fe²⁺. The combined biochemical and paleogeological data are consistent with a role for Fe²⁺ in an RNA World. RNA and Fe²⁺ could, in principle, support an array of RNA structures and catalytic functions more diverse than RNA with Mg²⁺ alone.
Conflict of interest statement
Figures
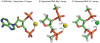

References
-
- Stein A, Crothers DM. Conformational changes of transfer RNA. The role of magnesium (II) equilibrium binding of magnesium (II) by Escherichia coli tRNAfMet. Biochemistry. 1976;15:160–168. - PubMed
-
- Lynch DC, Schimmel PR. Cooperative binding of magnesium to transfer ribonucleic acid studied by a fluorescent probe. Biochemistry. 1974;13:1841–1852. - PubMed
-
- Brion P, Westhof E. Hierarchy and dynamics of RNA folding. Annu Rev Biophys Biomol Struct. 1997;26:113–137. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

