Active zone protein Bassoon co-localizes with presynaptic calcium channel, modifies channel function, and recovers from aging related loss by exercise
- PMID: 22701595
- PMCID: PMC3368936
- DOI: 10.1371/journal.pone.0038029
Active zone protein Bassoon co-localizes with presynaptic calcium channel, modifies channel function, and recovers from aging related loss by exercise
Abstract
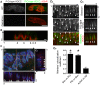
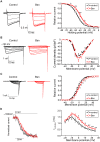
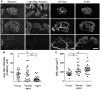
The P/Q-type voltage-dependent calcium channels (VDCCs) are essential for synaptic transmission at adult mammalian neuromuscular junctions (NMJs); however, the subsynaptic location of VDCCs relative to active zones in rodent NMJs, and the functional modification of VDCCs by the interaction with active zone protein Bassoon remain unknown. Here, we show that P/Q-type VDCCs distribute in a punctate pattern within the NMJ presynaptic terminals and align in three dimensions with Bassoon. This distribution pattern of P/Q-type VDCCs and Bassoon in NMJs is consistent with our previous study demonstrating the binding of VDCCs and Bassoon. In addition, we now show that the interaction between P/Q-type VDCCs and Bassoon significantly suppressed the inactivation property of P/Q-type VDCCs, suggesting that the Ca(2+) influx may be augmented by Bassoon for efficient synaptic transmission at NMJs. However, presynaptic Bassoon level was significantly attenuated in aged rat NMJs, which suggests an attenuation of VDCC function due to a lack of this interaction between VDCC and Bassoon. Importantly, the decreased Bassoon level in aged NMJs was ameliorated by isometric strength training of muscles for two months. The training increased Bassoon immunoreactivity in NMJs without affecting synapse size. These results demonstrated that the P/Q-type VDCCs preferentially accumulate at NMJ active zones and play essential role in synaptic transmission in conjunction with the active zone protein Bassoon. This molecular mechanism becomes impaired by aging, which suggests altered synaptic function in aged NMJs. However, Bassoon level in aged NMJs can be improved by muscle exercise.
Conflict of interest statement
Figures
Similar articles
-
Calcium channels link the muscle-derived synapse organizer laminin β2 to Bassoon and CAST/Erc2 to organize presynaptic active zones.J Neurosci. 2011 Jan 12;31(2):512-25. doi: 10.1523/JNEUROSCI.3771-10.2011. J Neurosci. 2011. PMID: 21228161 Free PMC article.
-
Dual-color STED microscopy reveals a sandwich structure of Bassoon and Piccolo in active zones of adult and aged mice.Sci Rep. 2016 Jun 20;6:27935. doi: 10.1038/srep27935. Sci Rep. 2016. PMID: 27321892 Free PMC article.
-
Active zones of mammalian neuromuscular junctions: formation, density, and aging.Ann N Y Acad Sci. 2012 Dec;1274(1):24-32. doi: 10.1111/j.1749-6632.2012.06836.x. Ann N Y Acad Sci. 2012. PMID: 23252894 Free PMC article. Review.
-
Differential Ca2+-dependence of transmitter release mediated by P/Q- and N-type calcium channels at neonatal rat neuromuscular junctions.Eur J Neurosci. 2002 Jun;15(12):1874-80. doi: 10.1046/j.1460-9568.2002.02015.x. Eur J Neurosci. 2002. PMID: 12099893
-
Calcium channels involved in neurotransmitter release at adult, neonatal and P/Q-type deficient neuromuscular junctions (Review).Mol Membr Biol. 2002 Oct-Dec;19(4):293-300. doi: 10.1080/0968768021000035087. Mol Membr Biol. 2002. PMID: 12512776 Review.
Cited by
-
Presynaptic [Ca(2+)] and GCAPs: aspects on the structure and function of photoreceptor ribbon synapses.Front Mol Neurosci. 2014 Feb 6;7:3. doi: 10.3389/fnmol.2014.00003. eCollection 2014. Front Mol Neurosci. 2014. PMID: 24567702 Free PMC article. Review.
-
Dependence-induced increase of alcohol self-administration and compulsive drinking mediated by the histone methyltransferase PRDM2.Mol Psychiatry. 2017 Dec;22(12):1746-1758. doi: 10.1038/mp.2016.131. Epub 2016 Aug 30. Mol Psychiatry. 2017. PMID: 27573876 Free PMC article.
-
Multiple roles of integrin-α3 at the neuromuscular junction.J Cell Sci. 2017 May 15;130(10):1772-1784. doi: 10.1242/jcs.201103. Epub 2017 Apr 6. J Cell Sci. 2017. PMID: 28386022 Free PMC article.
-
Effects of disease-afflicted and aging neurons on the musculoskeletal system.Bone. 2019 May;122:31-37. doi: 10.1016/j.bone.2019.01.023. Epub 2019 Jan 26. Bone. 2019. PMID: 30695738 Free PMC article. Review.
-
Segmentation of the mouse fourth deep lumbrical muscle connectome reveals concentric organisation of motor units.J Physiol. 2013 Oct 1;591(19):4859-75. doi: 10.1113/jphysiol.2013.258087. Epub 2013 Aug 12. J Physiol. 2013. PMID: 23940381 Free PMC article.
References
-
- Robitaille R, Adler EM, Charlton MP. Strategic location of calcium channels at transmitter release sites of frog neuromuscular synapses. Neuron. 1990;5:773–779. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

