Osteoclast activated FoxP3+ CD8+ T-cells suppress bone resorption in vitro
- PMID: 22701612
- PMCID: PMC3368916
- DOI: 10.1371/journal.pone.0038199
Osteoclast activated FoxP3+ CD8+ T-cells suppress bone resorption in vitro
Abstract
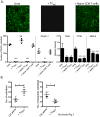
Background: Osteoclasts are the body's sole bone resorbing cells. Cytokines produced by pro-inflammatory effector T-cells (T(EFF)) increase bone resorption by osteoclasts. Prolonged exposure to the T(EFF) produced cytokines leads to bone erosion diseases such as osteoporosis and rheumatoid arthritis. The crosstalk between T-cells and osteoclasts has been termed osteoimmunology. We have previously shown that under non-inflammatory conditions, murine osteoclasts can recruit naïve CD8 T-cells and activate these T-cells to induce CD25 and FoxP3 (Tc(REG)). The activation of CD8 T-cells by osteoclasts also induced the cytokines IL-2, IL-6, IL-10 and IFN-γ. Individually, these cytokines can activate or suppress osteoclast resorption.
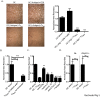
Principal findings: To determine the net effect of Tc(REG) on osteoclast activity we used a number of in vitro assays. We found that Tc(REG) can potently and directly suppress bone resorption by osteoclasts. Tc(REG) could suppress osteoclast differentiation and resorption by mature osteoclasts, but did not affect their survival. Additionally, we showed that Tc(REG) suppress cytoskeletal reorganization in mature osteoclasts. Whereas induction of Tc(REG) by osteoclasts is antigen-dependent, suppression of osteoclasts by Tc(REG) does not require antigen or re-stimulation. We demonstrated that antibody blockade of IL-6, IL-10 or IFN-γ relieved suppression. The suppression did not require direct contact between the Tc(REG) and osteoclasts.
Significance: We have determined that osteoclast-induced Tc(REG) can suppress osteoclast activity, forming a negative feedback system. As the CD8 T-cells are activated in the absence of inflammatory signals, these observations suggest that this regulatory loop may play a role in regulating skeletal homeostasis. Our results provide the first documentation of suppression of osteoclast activity by CD8 regulatory T-cells and thus, extend the purview of osteoimmunology.
Conflict of interest statement
Figures

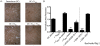

Similar articles
-
Osteoclast-Primed Foxp3+ CD8 T Cells Induce T-bet, Eomesodermin, and IFN-γ To Regulate Bone Resorption.J Immunol. 2016 Aug 1;197(3):726-35. doi: 10.4049/jimmunol.1600253. Epub 2016 Jun 20. J Immunol. 2016. PMID: 27324129 Free PMC article.
-
A Bone Anabolic Effect of RANKL in a Murine Model of Osteoporosis Mediated Through FoxP3+ CD8 T Cells.J Bone Miner Res. 2015 Aug;30(8):1508-22. doi: 10.1002/jbmr.2472. Epub 2015 May 21. J Bone Miner Res. 2015. PMID: 25656537 Free PMC article.
-
Osteoclast-induced Foxp3+ CD8 T-cells limit bone loss in mice.Bone. 2013 Sep;56(1):163-73. doi: 10.1016/j.bone.2013.05.024. Epub 2013 Jun 10. Bone. 2013. PMID: 23756229 Free PMC article.
-
Osteoclasts, rheumatoid arthritis, and osteoimmunology.Curr Opin Rheumatol. 2006 Jul;18(4):419-26. doi: 10.1097/01.bor.0000231912.24740.a5. Curr Opin Rheumatol. 2006. PMID: 16763464 Review.
-
Impact of cytokines and T lymphocytes upon osteoclast differentiation and function.Arthritis Res Ther. 2007;9(2):103. doi: 10.1186/ar2141. Arthritis Res Ther. 2007. PMID: 17381830 Free PMC article. Review.
Cited by
-
Monocyte Heterogeneity: Consequences for Monocyte-Derived Immune Cells.J Immunol Res. 2016;2016:1475435. doi: 10.1155/2016/1475435. Epub 2016 Jul 11. J Immunol Res. 2016. PMID: 27478854 Free PMC article. Review.
-
Osteoclast-Primed Foxp3+ CD8 T Cells Induce T-bet, Eomesodermin, and IFN-γ To Regulate Bone Resorption.J Immunol. 2016 Aug 1;197(3):726-35. doi: 10.4049/jimmunol.1600253. Epub 2016 Jun 20. J Immunol. 2016. PMID: 27324129 Free PMC article.
-
Tumor Microenvironment, Clinical Features, and Advances in Therapy for Bone Metastasis in Gastric Cancer.Cancers (Basel). 2022 Oct 6;14(19):4888. doi: 10.3390/cancers14194888. Cancers (Basel). 2022. PMID: 36230816 Free PMC article. Review.
-
The Multiple Biological Functions of Dipeptidyl Peptidase-4 in Bone Metabolism.Front Endocrinol (Lausanne). 2022 May 2;13:856954. doi: 10.3389/fendo.2022.856954. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 35586625 Free PMC article. Review.
-
Immune Function and Diversity of Osteoclasts in Normal and Pathological Conditions.Front Immunol. 2019 Jun 19;10:1408. doi: 10.3389/fimmu.2019.01408. eCollection 2019. Front Immunol. 2019. PMID: 31275328 Free PMC article. Review.
References
-
- Henriksen K, Neutzsky-Wulff AV, Bonewald LF, Karsdal MA. Local communication on and within bone controls bone remodeling. Bone. 2009;44:1026–1033. - PubMed
-
- Udagawa N, Takahashi N, Jimi E, Matsuzaki K, Tsurukai T, et al. Osteoblasts/stromal cells stimulate osteoclast activation through expression of osteoclast differentiation factor/RANKL but not macrophage colony-stimulating factor: receptor activator of NF-kappa B ligand. Bone. 1999;25:517–523. - PubMed
-
- Takami M, Woo JT, Nagai K. Osteoblastic cells induce fusion and activation of osteoclasts through a mechanism independent of macrophage-colony-stimulating factor production. Cell Tissue Res. 1999;298:327–334. - PubMed
-
- Suda T, Takahashi N, Udagawa N, Jimi E, Gillespie MT, et al. Modulation of osteoclast differentiation and function by the new members of the tumor necrosis factor receptor and ligand families. Endocr Rev. 1999;20:345–357. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

