Olopatadine ophthalmic solution suppresses substance P release in the conjunctivitis models
- PMID: 22701861
- PMCID: PMC3345324
- DOI: 10.5415/apallergy.2012.2.2.115
Olopatadine ophthalmic solution suppresses substance P release in the conjunctivitis models
Abstract
Background: Olopatadine hydrochloride ophthalmic solutions are treated for allergic conjunctival diseases that are a selective histamine H1 receptor antagonist and an inhibitor of the release of mediators including histamine from the human mast cells. Substance P (SP) levels are increased in tears of patients with allergic conjunctivitis. However, little is known about the regulation of SP release by anti-allergic ophthalmic solutions.
Objective: We investigated that the effect of olopatadine hydrochloride ophthalmic solutions (olopatadine 0.1% and olopatadine 0.2%) on rat conjunctivitis models compared with other anti-allergic ophthalmic solutions.
Methods: Conjunctivitis was induced by subconjunctival injection of histamine or intravenous injection of ovalbumin in rats passively sensitized with anti-ovalbumin anti-serum. The releases of SP were determined in the conjunctiva and tears using rat antigen-induced conjunctivitis models.
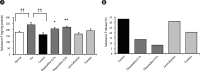
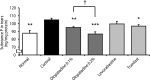
Results: Olopatadine 0.1% and 0.2% significantly inhibited the increased conjunctival dye leaked in the histamine- or antigen-induced hyperpermeability. The inhibitory effects by olopatadine were more potent than by other tested anti-allergic ophthalmic solutions. Moreover, olopatadine significantly inhibited the release of SP from the conjunctiva.
Conclusion: These results indicate that olopatadine ophthalmic solutions appear to exert additional SP release inhibition besides dual-action such as selective histamine H1 receptor antagonistic action and mast cell stabilization action.
Keywords: Allergic conjunctivitis; Olopatadine hydrochloride; Ophthalmic solution; Rat; Substance P.
Figures


References
-
- Sharif NA, Xu SX, Miller ST, Gamache DA, Yanni JM. Characterization of the ocular antiallergic and antihistaminic effects of olopatadine (AL-4943A), a novel drug for treating ocular allergic diseases. J Pharmacol Exp Ther. 1996;278:1252–1261. - PubMed
-
- Yanni JM, Stephens DJ, Miller ST, Weimer LK, Graff G, Parnell D, Lang LS, Spellman JM, Brady MT, Gamache DA. The in vitro and in vivo ocular pharmacology of olopatadine (AL-4943A), an effective anti-allergic/antihistaminic agent. J Ocul Pharmacol Ther. 1996;12:389–400. - PubMed
-
- Yanni JM, Miller ST, Gamache DA, Spellman JM, Xu S, Sharif NA. Comparative effects of topical ocular anti-allergy drugs on human conjunctival mast cells. Ann Allergy Asthma Immunol. 1997;79:541–545. - PubMed
-
- Yanni JM, Weimer LK, Sharif NA, Xu SX, Gamache DA, Spellman JM. Inhibition of histamine-induced human conjunctival epithelial cell responses by ocular allergy drugs. Arch Ophthalmol. 1999;117:643–647. - PubMed
-
- Fujishima H, Takeyama M, Takeuchi T, Saito I, Tsubota K. Elevated levels of substance P in tears of patients with allergic conjunctivitis and vernal keratoconjunctivitis. Clin Exp Allergy. 1997;27:372–378. - PubMed
LinkOut - more resources
Full Text Sources
