New insights into osteoporosis: the bone-fat connection
- PMID: 22702419
- PMCID: PMC3634716
- DOI: 10.1111/j.1365-2796.2012.02564.x
New insights into osteoporosis: the bone-fat connection
Abstract
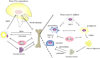
Osteoporosis and obesity are chronic disorders that are both increasing in prevalence. The pathophysiology of these conditions is multifactorial and includes genetic, environmental and hormonal determinants. Although it has long been considered that these are distinct disorders rarely found in the same individual, emerging evidence from basic and clinical studies support an important interaction between adipose tissue and the skeleton. It is proposed that adiposity may influence bone remodelling through three mechanisms: (i) secretion of cytokines that directly target bone, (ii) production of adipokines that influence the central nervous system thereby changing sympathetic impulses to bone and (iii) paracrine influences on adjacent skeletal cells. Here we focus on the current understanding of bone-fat interactions and the clinical implications of recent studies linking obesity to osteoporosis.
© 2012 The Association for the Publication of the Journal of Internal Medicine.
Figures


References
-
- Karsenty G. Convergence between bone and energy homeostases: leptin regulation of bone mass. Cell Metab. 2006;4:341–348. - PubMed
-
- Ryden M, Arner P. Tumour necrosis factor-alpha in human adipose tissue -- from signalling mechanisms to clinical implications. J Intern Med. 2007;262:431–438. - PubMed
-
- Langsted A, Freiberg JJ, Tybjaerg-Hansen A, Schnohr P, Jensen GB, Nordestgaard BG. Nonfasting cholesterol and triglycerides and association with risk of myocardial infarction and total mortality: the Copenhagen City Heart Study with 31 years of follow-up. J Intern Med. 2011;270:65–75. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

