Complete absence of the αGal xenoantigen and isoglobotrihexosylceramide in α1,3galactosyltransferase knock-out pigs
- PMID: 22702471
- PMCID: PMC3614413
- DOI: 10.1111/j.1399-3089.2012.00705.x
Complete absence of the αGal xenoantigen and isoglobotrihexosylceramide in α1,3galactosyltransferase knock-out pigs
Abstract
Background: Anti-Galα1,3Galβ-R natural antibodies are responsible for hyperacute rejection in pig-to-primate xenotransplantation. Although the generation of pigs lacking the α1,3galactosyltransferase (GalT) has overcome hyperacute rejection, antibody-mediated rejection is still a problem. It is possible that other enzymes synthesize antigens similar to Galα1,3Gal epitopes that are recognized by xenoreactive antibodies. The glycosphingolipid isoglobotrihexosylceramide (iGb₃) represents such a candidate expressing an alternative Galα1,3Gal epitope. The present work determined whether the terminal Galα1,3Gal disaccharide is completely absent in Immerge pigs lacking the GalT using several different highly sensitive methods.
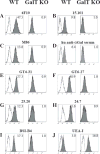
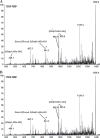
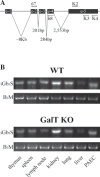
Methods: The expression of Galα1,3Gal was evaluated using a panel of antibodies and lectins by flow cytometry and fluorescent microscopy; GalT activity was detected by an enzymatic assay; and ion trap mass spectroscopy of neutral cellular membranes extracted from aortic endothelial was used for the detection of sugar structures. Finally, the presence of iGb₃ synthase mRNA was tested by RT-PCR in pig thymus, spleen, lymph node, kidney, lung, and liver tissue samples.
Results: Aortic endothelial cells derived from GalT knockout pigs expressed neither Galα1,3Gal nor iGb₃ on their surface, and GalT enzymatic activity was also absent. Lectin staining showed an increase in the blood group H-type sugar structures present in GalT knockout cells as compared to wild-type pig aortic endothelial cells (PAEC). Mass spectroscopic analysis did not reveal Galα1,3Gal in membranes of GalT knockout PAEC; iGb₃ was also totally absent, whereas a fucosylated form of iGb₃ was detected at low levels in both pig aortic endothelial cell extracts. Isoglobotrihexosylceramide 3 synthase mRNA was expressed in all pig tissues tested whether derived from wild-type or GalT knockout animals.
Conclusions: These results confirm unequivocally the absence of terminal Galα1,3Gal disaccharides in GalT knockout endothelial cells. Future work will have to focus on other mechanisms responsible for xenograft rejection, in particular non-Galα1,3Gal antibodies and cellular responses.
© 2012 John Wiley & Sons A/S.
Conflict of interest statement
DZ is a consultant for BioTex, Houston, TX, and an inventor involved in patents related to technologies mentioned in this article, issued or in application.
Figures


References
-
- Tai HC, Zhu X, Hara H, et al. The pig-to-primate immune response: relevance for xenotransplantation. Xenotransplantation. 2007;14:227–235. - PubMed
-
- Li S, Waer M, Billiau AD. Xenotransplantation: role of natural immunity. Transpl Immunol. 2009;21:70–74. - PubMed
-
- Galili U, Shohet SB, Kobrin E, Stults CL, Macher BA. Man, apes, and Old World monkeys differ from other mammals in the expression of alpha-galactosyl epitopes on nucleated cells. J Biol Chem. 1988;263:17755–17762. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

