Elacytarabine has single-agent activity in patients with advanced acute myeloid leukaemia
- PMID: 22702906
- PMCID: PMC4191720
- DOI: 10.1111/j.1365-2141.2012.09186.x
Elacytarabine has single-agent activity in patients with advanced acute myeloid leukaemia
Abstract
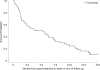
Elacytarabine is a novel cytotoxic nucleoside analogue, independent of nucleoside transporters (e.g. human Equilibrative Nucleoside Transporter 1 [hENT1]) for cell uptake, and mechanisms of action similar to those of cytarabine. This Phase II study assessed the efficacy and safety of elacytarabine in patients with advanced stage acute myeloid leukaemia (AML). Patients received 2000 mg/m(2) per d continuously i.v. during days 1-5 every 3 weeks. Patients were matched by six risk factors with historical controls; remission rate (assessed after 1 or 2 cycles) and 6-month survival were compared. Sixty-one patients, median age 58 years, were enrolled; 52% had five or six risk factors. The remission rate was 18% (95% confidence interval: 9-30%) vs. 4% in controls (P < 0·0001), 6-month survival rate was 43%, median overall survival was 5·3 months (vs. 1·5 months); 10 patients (16%) were referred for stem cell transplantation after treatment. Side effects were predictable and manageable. The most common grade 3/4 non-haematological adverse events were febrile neutropenia, hypokalemia, fatigue, hyponatraemia, dyspnoea and pyrexia. Thirty-day all-cause mortality, after start of treatment, was 13% vs. 25% in controls. Elacytarabine has monotherapy activity in patients with advanced AML. This study provides proof-of-concept that lipid esterification of nucleoside analogues is clinically relevant.
© 2012 Blackwell Publishing Ltd.
Figures

Similar articles
-
A phase II study of elacytarabine in combination with idarubicin and of human equilibrative nucleoside transporter 1 expression in patients with acute myeloid leukemia and persistent blasts after the first induction course.Leuk Lymphoma. 2014 Sep;55(9):2114-9. doi: 10.3109/10428194.2013.867489. Epub 2014 Jan 24. Leuk Lymphoma. 2014. PMID: 24255981 Clinical Trial.
-
Elacytarabine (CP-4055) in the treatment of acute myeloid leukemia.Future Oncol. 2013 Aug;9(8):1073-82. doi: 10.2217/fon.13.130. Future Oncol. 2013. PMID: 23902239
-
Quizartinib versus salvage chemotherapy in relapsed or refractory FLT3-ITD acute myeloid leukaemia (QuANTUM-R): a multicentre, randomised, controlled, open-label, phase 3 trial.Lancet Oncol. 2019 Jul;20(7):984-997. doi: 10.1016/S1470-2045(19)30150-0. Epub 2019 Jun 4. Lancet Oncol. 2019. PMID: 31175001 Clinical Trial.
-
Clinical potential of elacytarabine in patients with acute myeloid leukemia.Ther Adv Hematol. 2014 Dec;5(6):211-20. doi: 10.1177/2040620714552615. Ther Adv Hematol. 2014. PMID: 25469211 Free PMC article. Review.
-
[Low dose cytarabine in the treatment of acute myeloid leukemia. Apropos of 41 cases].Bull Cancer. 1996 Dec;83(12):996-1001. Bull Cancer. 1996. PMID: 9116380 Review. French.
Cited by
-
Advances in the development of nucleoside and nucleotide analogues for cancer and viral diseases.Nat Rev Drug Discov. 2013 Jun;12(6):447-64. doi: 10.1038/nrd4010. Nat Rev Drug Discov. 2013. PMID: 23722347 Review.
-
The Novel Phospholipid Mimetic KPC34 Is Highly Active Against Acute Myeloid Leukemia with Activated Protein Kinase C.Transl Oncol. 2020 Jul;13(7):100780. doi: 10.1016/j.tranon.2020.100780. Epub 2020 May 16. Transl Oncol. 2020. PMID: 32428837 Free PMC article.
-
Electrolyte Disorders Induced by Antineoplastic Drugs.Front Oncol. 2020 May 19;10:779. doi: 10.3389/fonc.2020.00779. eCollection 2020. Front Oncol. 2020. PMID: 32509580 Free PMC article. Review.
-
Improving nucleoside analogs via lipid conjugation: Is fatter any better?Crit Rev Oncol Hematol. 2016 Apr;100:46-56. doi: 10.1016/j.critrevonc.2016.01.015. Epub 2016 Jan 21. Crit Rev Oncol Hematol. 2016. PMID: 26829896 Free PMC article. Review.
-
Novel therapeutics in acute myeloid leukemia.Curr Hematol Malig Rep. 2014 Jun;9(2):109-17. doi: 10.1007/s11899-014-0199-0. Curr Hematol Malig Rep. 2014. PMID: 24633743 Review.
References
-
- Adema AD, Laan AC, Myhren F, Fichtner I, Verheul HM, Sandvold ML, Peters GJ. Cell cycle effects of fatty acid derivatives of cytarabine, CP-4055, and of gemcitabine, CP-4126, as basis for the interaction with oxaliplatin and docetaxel. International Journal of Oncology. 2010a;36:285–294. - PubMed
-
- Adema AD, Losekoot N, Smid K, Kathmann I, Myhren F, Sandvold ML, Peters GJ. Induction of resistance to the lipophilic cytarabine prodrug elacytarabine (CP-4055) in CEM leukemic cells. Nucleosides Nucleotides Nucleic Acids. 2010b;29:394–399. - PubMed
-
- Becker PS, Kantarjian HM, Appelbaum FR, Petersdorf SH, Storer B, Pierce S, Shan J, Hendrie PC, Pagel JM, Shustov AR, Stirewalt DL, Faderl S, Harrington E, Estey EH. Clofarabine with high dose cytarabine and granulocyte colony-stimulating factor (G-CSF) priming for relapsed and refractory acute myeloid leukaemia. British Journal of Haematology. 2011;155:182–189. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

