Zinc thiolate reactivity toward nitrogen oxides: insights into the interaction of Zn2+ with S-nitrosothiols and implications for nitric oxide synthase
- PMID: 22702952
- PMCID: PMC3389809
- DOI: 10.1021/ic3007684
Zinc thiolate reactivity toward nitrogen oxides: insights into the interaction of Zn2+ with S-nitrosothiols and implications for nitric oxide synthase
Abstract
Zinc thiolate complexes containing N(2)S tridentate ligands were prepared to investigate their reactivity toward reactive nitrogen species, chemistry proposed to occur at the zinc tetracysteine thiolate site of nitric oxide synthase (NOS). The complexes are unreactive toward nitric oxide (NO) in the absence of dioxygen, strongly indicating that NO cannot be the species directly responsible for S-nitrosothiol formation and loss of Zn(2+) at the NOS dimer interface in vivo. S-Nitrosothiol formation does occur upon exposure of zinc thiolate solutions to NO in the presence of air, however, or to NO(2) or NOBF(4), indicating that these reactive nitrogen/oxygen species are capable of liberating zinc from the enzyme, possibly through generation of the S-nitrosothiol. Interaction between simple Zn(2+) salts and preformed S-nitrosothiols leads to decomposition of the -SNO moiety, resulting in release of gaseous NO and N(2)O. The potential biological relevance of this chemistry is discussed.
Figures





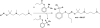
References
-
- Bredt DS, Snyder SH. Annu. Rev. Biochem. 1994;63:175–195. - PubMed
-
- Lehninger AL, Nelson DL, Cox MM. Lehninger Principles of Biochemistry. 4 ed W. H. Freeman & Co.; 2004.
-
- Moncada S, Palmer RMJ, Higgs EA. Pharmacol. Rev. 1991;43:109–142. - PubMed
-
- Nathan C, Xie Q.-w. Cell. 1994;78:915–918. - PubMed
-
- Ghosh DK, Stuehr DJ. Biochemistry. 1995;34:801–807. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

